- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Battle of Batteries
Battery or cells are referred to as the parallel combination of electrochemical cells. The major difference between a primary cell and the secondary cell is that primary cells are the ones that cannot be charged but secondary cells are the ones that are rechargeable.
Primary cells have high density and get discharged slowly. Since there is no fluid inside these cells they are also known as dry cells. The internal resistance is high and the chemical reaction is irreversible. Its initial cost is cheap and also primary cells are easy to use.
1. Daniel cell : Daniel cell is a primary cell which cannot supply steady current for a long time. It consists of a copper vessel containing a strong solution of copper sulphate. A zinc rod is dipped in dilute sulphuric acid contained in a porous pot. The porous pot is placed inside the copper sulphate solution.
The zinc rod reacting with dilute sulphuric acid produces Zn++ ions and 2 electrons. Zn++ ions pass through the pores of the porous pot and reacts with copper sulphate solution, producing Cu++ ions. The Cu++ ions deposit on the copper vessel. When Daniel cell is connected in a circuit, the two electrons on the zinc rod pass through the external circuit and reach the copper vessel thus neutralizing the copper ions. This constitutes an electric current from copper to zinc. Daniel cell produces an emf of 1.08 volt.
2. Leclanche cell: A Leclanche cell consists of a carbon electrode packed in a porous pot containing manganese dioxide and charcoal powder. The porous pot is immersed in a saturated solution of ammonium chloride (electrolyte) contained in an outer glass vessel. A zinc rod is immersed in electrolytic solution.
At the zinc rod, due to oxidation reaction Zn atom is converted into Zn++ ions and 2 electrons. Zn++ ions reacting with ammonium chloride produces zinc chloride and ammonia gas.
i.e Zn++ + 2 NH4Cl - 2NH3 + ZnCl2 + 2 H+ + 2e-
The ammonia gas escapes. The hydrogen ions diffuse through the pores of the porous pot and react with manganese dioxide. In this process the positive charge of hydrogen ion is transferred to carbon rod. When zinc rod and carbon rod are connected externally, the two electrons from the zinc rod move towards carbon and
neutralizes the positive charge. Thus current flows from carbon to zinc. Leclanche cell is useful for supplying intermittent current. The emf of the cell is about 1.5 V, and it can supply a current of 0.25 A.
3.Voltaic / Galvanic cell: A simple voltaic cell is made by immersing one zinc plate and one copper plate inside a water diluted sulfuric acid solution. If the copper plate and zinc plate are connected externally with an electrical load, an electric current starts flowing from copper plate to zinc plate through the load. That means there is some electrical potential difference developed between the copper plate and the zinc plate. As the current flows from copper to zinc, it is obvious that the copper plate becomes positively charged and the zinc plate becomes negatively charged.
Secondary cells have low energy density and are made of molten salts and wet cells. The internal resistance is low and the chemical reaction is reversible. Its initial cost is high and is a little complicated to use when compared to the primary cell.
The growing concern over climate change has led to global efforts to electrify the transportation sector. In parallel, cost of Li-ion (Lithium-ion) battery technology has decreased by a staggering order of magnitude in the last decade. The convergence of these two factors has resulted in a unique time in our history where we are at the cusp of a dramatic transition in the transportation sector, with electric vehicles poised to replace petrol vehicles. EVs are vehicles that are either partially or fully powered on electric power. While some EVs used lead acid or nickel metal hydride batteries, the standard for modern battery electric vehicles is now considered to be lithium ion batteries.
The world governments have been providing incentives to usher in the transition and private industry ramping up plans for capturing the market. There is a worldwide race emerging, with vehicle companies, battery manufacturers, and material suppliers vying with each other for market share. However, Li-ion batteries are complex devices requiring a level of sophistication that can takes years to perfect. Hurrying the development of this complex technology without careful safeguards can lead to increasing safety incidents, as evidenced recently on Indian roads.
How does a lithium-ion battery work?
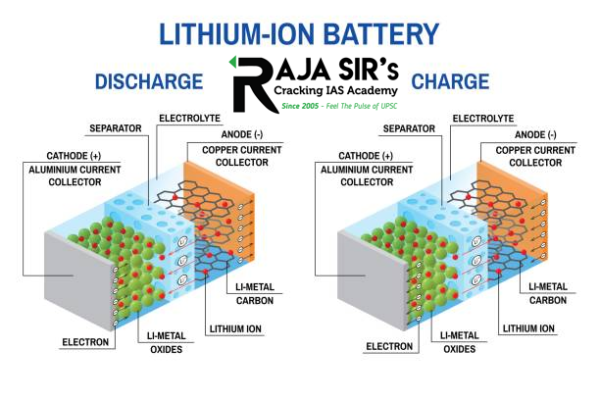
- Anode, cathode, electrolyte and separator are the main components of a lithium-ion (rechargeable) battery.
- The two electrodes are immersed in the electrolyte and are separated by the separator.
- The anode is usually made up of graphite (carbon).
- Carbon graphite has a layered structure that can store the lithium ions in between its layers.
- The cathode is made up of a combination of lithium-cobalt.
- Lithium is unstable in the element form; hence the combination lithium-cobalt oxide is used for the cathode.
- Cathode plays an important role in determining the energy density of a Li-ion battery.
- The higher amount of lithium, bigger the capacity.
Working of a lithium-ion battery
- Both electrodes in a li-ion battery can intercalate or ‘absorb’ lithium ions.
- When the battery is being charged, lithium ions are absorbed (stored) in the anode.
- During discharge, lithium ions naturally flow back to the cathode through the electrolyte.
- This creates free electrons in the anode which move along the wire generating electricity.
- The process (to and fro movement of lithium-ion) repeats with each charge and discharge cycles.
- Electrolyte (lithium salt) enables the movement of lithium ions between the electrodes. Most of the electrolytes used in commercial lithium-ion batteries are non-aqueous solutions, in which Lithium hexafluorophosphate (LiPF6) salt dissolved in organic carbonates, in particular, mixtures of ethylene carbonate (EC) with dimethyl carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC), and/or ethyl methyl carbonate (EMC).
|
Charge Process: Positive electrode (cathode) is oxidized (loses electrons) and Li+ ions pass across the electrolyte and are intercalated (insert between layers) in negative electrode (anode – graphite). Discharge Process (opposite of charge process): An oxidation reaction occurs at the anode (-ve), Li+ ions are de-intercalated and migrate across the electrolyte to be re-intercalated into the cathode material. |
- The separator functions as a physical barrier keeping cathode and anode apart.
- It prevents the direct flow of electrons and lets only the ions pass through.
- While the cathode determines the performance of a battery, electrolyte and separator determine its safety.
- Permeable polymer membranes such as polyethylene (PE) and polypropylene (PP) are used as separators.
Battery manufacturing is a complex operation involving forming sheets of the anode and cathode and assembling them into a sandwich structure held apart by a thin separator.
Separators, about 15 microns in thickness — about a fifth of the thickness of the human hair — perform the critical function of preventing the anode and cathode from shorting. Accidental shorting of the electrodes is a known cause of fires in Li-ion cells. It is important that the various layers are assembled with high precision with tight tolerances maintained throughout the manufacturing process. Safety features, such as thermal switches that turn off if the battery overheats, are added as the sandwich is packaged into a battery cell.
Battery cells are assembled into modules and then further assembled into packs. Li-ion batteries require tight control on the state of charge and the temperature of operation to enhance safety and increase usable life, achieved by adding multiple sensors. Packs are designed to ensure uniform temperature profile with minimal thermal variation during operation. Ensuring robust detection, coupled with battery management systems that interpret the data and change operation based on changes to the batteries state, remain critically important in enhancing battery performance.
There are multiple tradeoffs in this complex ecosystem: engineering higher safety often results in higher costs and lower driving range. In this competitive landscape where companies are vying for market share, a race to the bottom can compromise safety.
What causes battery fires?
While Li-ion batteries are complex, over the last three decades numerous companies have perfected the art of manufacturing high-quality cells and integrating them into vehicles with minimal safety concerns. The energy density of petrol is five hundred times that of a typical Li-ion battery, therefore safety should be manageable if robust controls are in place. However, batteries do store energy in a small package and if the energy is released in an uncontrolled fashion, the thermal event can be significant.
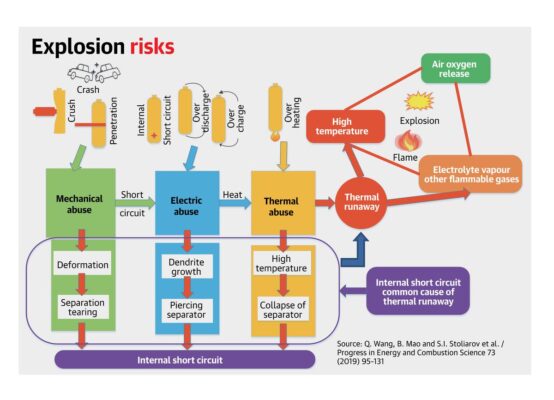
Battery fires, like other fires, occur due to the convergence of three parts of the “fire triangle”: heat, oxygen, and fuel. If an adverse event such as a short circuit occurs in the battery, the internal temperature can raise as the anode and cathode release their energy through the short. This, in turn, can lead to a series of reactions from the battery materials, especially the cathode, that release heat in an uncontrolled manner, along with oxygen.
Such events also rupture the sealed battery further exposing the components to outside air and the second part of the fire triangle, namely, oxygen. The final component of the triangle is the liquid electrolyte, which is flammable and serves as a fuel. The combination leads to catastrophic failure of the battery resulting in smoke, heat, and fire, released instantaneously and explosively.
The trigger for such events can be a result of internal shorts (like a manufacturing defect that results in sharp objects penetrating the separator), external events (an accident leading to puncture of the cell and shorting of the electrodes), overcharging the battery which leads to heat releasing reactions on the cathode ( by a faulty battery management system that does not shut down charging despite the battery achieving its designed charge state), or bad thermal design at the module and pack level (by not allowing the battery internal heat to be released). Any of these triggers may cascade into a significant safety incident.
Are battery fires inevitable?.
Over the past three decades, Li-ion batteries have proved to be extremely safe, with the industry increasing controls as safety incidents have surfaced. Safety is a must and is an important consideration that battery and vehicle manufacturers can design for at multiple levels from the choice of battery material to designs at the cell, pack, and vehicle level.
Preventing fires requires breaking the fire triangle. Battery cathodes are a leading cause of the heat release. Some cathodes, such as ones with lower nickel content or moving to iron phosphate, can increase safety. Tightly controlled manufacturing will prevent accidental shorts in the cells, eliminating a leading cause of fires. Many companies now add a ceramic layer on the separator to mechanically prevent shorts. Sensing the state of the battery and integrating this data into sophisticated battery management systems is an important aspect of design. Protecting the cell with robust thermal management is critical, especially in India where ambient temperatures are high. Finally, battery packs need to be protected from external penetration. Any large-scale manufacturing process inevitably has a certain percentage of defects; therefore, such steps are needed to minimise the number of adverse events.
Long term changes are also underway. Safety remains a concern for Li-ion manufacturers worldwide especially as cell sizes become larger for applications like solar-connected storage. Companies are developing internal “switches” that turn off parts of the battery that undergo thermal events to stop them at their inception. Research is now underway to replace the flammable liquid electrolyte with a solid electrolyte to eliminate one part of the fire triangle. A similar thread of research is the development of nonflammable liquid electrolytes. All these changes promise to remove the threat of battery fires as the roll out of mass electrification takes place.
Iron ion battery developed by IIT Madras
- Fe2+ ions are the charge carriers in iron ion battery (in lithium-ion battery lithium ions do the job).
- The iron ion battery uses mild steel as the anode and Vanadium pentoxide as the cathode.
- The large inter-layer spacing in vanadium pentoxide makes intercalation easier (loss and gain of ions).
- In pure iron, intercalation is not possible. But, a small amount of carbon in mild steel facilitates this process.
- Ether-based electrolyte containing dissolved iron perchlorate is used as an electrolyte.
- The energy density of iron ion battery is 220 Wh/kg (350 Wh/kg in case of lithium-ion battery).
- When compared with lithium metal-based batteries, iron ion batteries would be cheaper yet safer.
Energy density is measured in watt-hours per kilogram (Wh/kg) and is the amount of energy the battery can store with respect to its mass.
How is iron better than lithium?
- The redox potential (potential to lose or gain electrons) of iron ion is higher than lithium-ion.
- The radius of the Fe2+ ion is nearly the same as that of the lithium-ion.
- Iron is more stable during the charging process and therefore prevents short-circuiting of the batteries.
- When more iron ions bind to the cathode, more energy (higher energy density) can be stored in the battery.
Comparison: Lead-acid battery, Lithium-ion battery & Iron ion battery by IIT
| Comparison table | Lead-acid battery | Lithium-ion battery | Iron ion battery by IIT |
| Electrolyte | Sulphuric acid | Lithium salt (Lithium hexafluorophosphate) | Iron perchlorate |
| Anode | Lead | Carbon (graphite) | Mild Steel |
| Cathode | Lead dioxide | Lithium-Cobalt Oxide (Lithium-Nickel-Manganese-Cobalt Oxide) | Vanadium pentoxide |
| Applications | Inverters, automobile batteries, solar batteries | Mobile, laptop, electric vehicle batteries | – |
| Energy Density (Wh/kg) | 30 to 40 | 350 | 220 |
| Weight and Space | Heavy and occupies more space | Comparatively lighter and occupies less space | – |
| Lifecycle | Low (2-4 years) | High (6-8 years) | – |
| Maintenance | Yes | No | No |
| Reliability | Low (full discharge damages battery) | High | – |
| Initial cost | Low | High | – |
| Lifecycle cost | High | Low | |
| Toxicity | High | Low | Low |
Lithium
Why lithium?
- Lithium is the lightest metal and a powerful reducing agent (willing to donate its electrons).
- Lithium-ion batteries capitalize on the strong reducing potential of lithium ions to power the redox reaction — reduction at the cathode, oxidation at the anode.
Among twelve minerals identified as strategic minerals, Lithium and Cobalt are significant.
- Lithium is lightest known metal. It has a density of 0.534 g/cm3 (half as dense as water).
- It’s light and soft and has the lowest melting points of all metals and a high boiling point.
- Lithium-ion batteries are key to lightweight, rechargeable power for laptops, phones, electric vehicles.
- Lithium and another battery component, cobalt, could become scarce as demand increases.
- China controls most of the lithium supply across the world.
| World’s Lithium Reserves in Million Tons | World’s Lithium Production in Thousand Tons | ||||
| Country | Reserves | Country | Production | ||
| Chile | 7.5 | 47% | Australia | 18.7 | 43% |
| China | 3.2 | 20% | Chile | 14.1 | 33% |
| Australia | 2.7 | 17% | Argentina | 5.5 | 13% |
| Argentina | 2 | 13% | China | 3 | 7% |
| World total | 16 MT | World total | 43 TT | ||
Cobalt
- Cobalt is an important ferromagnetic alloying metal having irreplaceable industrial applications.
- Cobalt is extracted as a by-product of copper, nickel, zinc or precious metals.
- Superalloys made of cobalt are wear & corrosion-resistant at elevated temperatures.
Role of cobalt in Lithium-ion batteries
- Lithium-cobalt-oxide is used as the cathode in rechargeable batteries.
- Lithium-cobalt-oxide is an intercalation compound with the lithium, cobalt and oxygen arranged in layers.
- Cobalt is indispensable to assure the rate performance (rate of charging & discharging occurs).
- When the lithium-ion arrives or departs from the cathode, cobalt changes its oxidation state (compensates for the gain/loss of charge) so that the lithium-cobalt-oxide stays electrically neutral.
- Cathodes are commonly oxides made from transition metals such as nickel, cobalt, copper, iron, etc.
- Replacing the costly cobalt with significantly cheaper nickel can be a fire hazard.
- Aluminium & manganese can be added to stabilize, but it lowers the capacity of the cell by a small amount.
Cobalt Reserves across India and the World
| State | Reserves in MT | Region with reserves | |
|
31 | 69% | Kendujhar and Jajpur districts |
|
9 | 20% | Singhbhum district |
|
5 | 11% | Tuensang district |
| Total | 44.9 MT | Presently, there is no production of cobalt from cobalt resources. | |
|
|||
- The demand for cobalt is usually met through imports.
- Recycling technologies for recovery of cobalt from waste Li-ion batteries have been an evolving process.
- Imports of cobalt and alloys were at 875 tonnes in 2017-18.
- Imports were mainly from USA & Canada (13% each), Belgium (12%), Norway & UK (9% each) and China (8%) & Morocco (7%).
| World’s Reserves of Cobalt Content (in TT) | World’s Production of Cobalt Content in 2017 (in TT) | ||||
| Country | Reserves | Country | Production | ||
| Congo (Kinshasa) | 3400 | 49% | Congo | 82.5 | 59% |
| Australia | 1200 | 17% | New Caledonia | 9.4 | 7% |
| Cuba | 500 | 7% | China | 9 | 6% |
| World Total | 6900 TT | Total | 139 TT | ||
Internal Combustion Engine Vehicles vs. Electric Vehicles
EVs are a lot better than ICEVs
| Internal Combustion Engine Vehicles (ICEV) | Electric Vehicles (EV) | Winner | |
| Major Components | IC engine, Transmission System. | 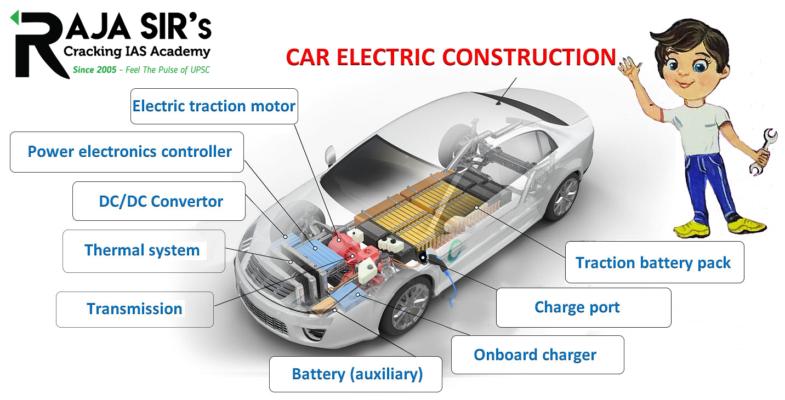 |
EV |
| Weight | Comparatively heavier. | Comparatively lighter. | EV |
| Heavy due to large and heavy metallic engines with complicated design. | Motor engines are relatively lighter as they have fewer components and simplistic design. | EV | |
| Space occupied by components | Comparatively more because of large engines. | Comparatively less ==> more space for seating ==> good for congested countries like India | EV |
| Efficiency | Less efficient because of loss of energy in the form of heat in IC engines and due to friction between transmission systems (rotatory motion has to be transmitted using a complex set of bearings and shafts). | More efficient as the loss of energy in the form of heat is very low (not many moving parts in motors) and transmission losses are minimum (the motor engine shaft transmits rotatory motion either directly to the wheels or with the help of fewer bearings and shafts). | EV |
| Maintenance | More maintenance (frequent, oil change, components replacement) is required as there are many moving parts. | Less maintenance as the battery is the only major component to be replaced. (low recurring cost) | EV |
| The initial cost of development and ownership | Comparatively low as the technology is in place for a century now. | High as the technology is still evolving. | IC |
| Total lifecycle economic cost | High | Low (electricity cost associated with operating an EV over a distance of 1 km is significantly lower than the petrol/diesel cost required to operate a comparable IC vehicle) | EV |
| Acceleration and speed control | Comparatively less as there many states like ignition, four stages of IC engine, transmission, etc. | EVs are much faster as the transmission of power and rotatory motion are almost instantaneous. | EV |
| Environmental footprint | High | Comparatively low (EV are more efficient) | |
| Range | Once the tank is full ICEVs can travel non-stop for hundreds of km | The range of EVs at present is only a few hundred km. | IC |
| Fuelling | Done in a few minutes. | Charging batteries take a few hours | IC |
| Infrastructure | Filling stations and other infrastructure is in place. | Charging stations are slowly popping up. | IC |
| Resale value | Resale value is falling as EVs are the future | Better | EV |
| Import-substitution. | Heavy dependence on imported fuels. | Clean electricity can replace fossil fuels.India now generates 22% (79 GW) of its electricity from renewable sources alone. | EV |
Lithium iron phosphate (LFP) batteries









 Latest News
Latest News
 General Studies
General Studies