- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Haber-Bosch Process and Food Supply
- The Haber-Bosch process, developed in the early 20th century, revolutionized global agriculture and food production by enabling the industrial synthesis of ammonia (NH3) from atmospheric nitrogen (N2) and hydrogen (H2).
- This innovation, which allowed for the mass production of nitrogen-based fertilizers, has had a profound impact on feeding the world’s growing population and supporting modern agriculture.
Understanding Nitrogen and Its Role in Nature
- Nitrogen (N2) is a critical element for life, as it is a key component of proteins, enzymes, and amino acids in both plants and animals.
- Although nitrogen is abundant in the atmosphere, making up about 78% of the air, it is mostly present in an inert form (N2) that cannot be directly utilized by plants.
- Plants require reactive nitrogen, such as ammonia (NH3) or nitrates (NO3-), to grow and thrive.
- In nature, reactive nitrogen is produced through two primary methods:
- Lightning: The immense energy from lightning breaks nitrogen molecules, allowing them to combine with oxygen to form nitrogen oxides, which later become nitrates in the soil.
- Nitrogen-Fixing Bacteria: Certain bacteria, such as Rhizobia, form symbiotic relationships with plants like legumes, helping convert atmospheric nitrogen into forms usable by plants.
- However, these natural processes provide limited amounts of nitrogen, insufficient to support the world’s growing demand for food.
The Haber-Bosch Process
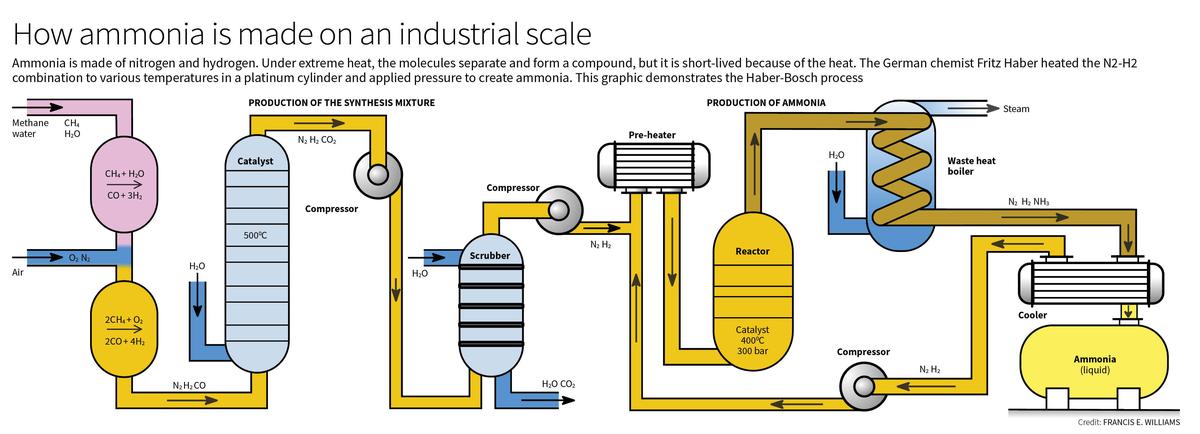
- The Haber-Bosch process, developed by Fritz Haber and later industrialized by Carl Bosch, provided a solution to this problem by creating an artificial method for nitrogen fixation.
- The process involves combining nitrogen (N2) and hydrogen (H2) under high pressure (around 200 atmospheres) and temperatures of about 400-500°C, in the presence of a catalyst, to produce ammonia.
- Ammonia produced through this process is a critical ingredient in synthetic fertilizers, which are used to enrich soil and promote plant growth.
- The availability of ammonia-based fertilizers has enabled the world’s agricultural productivity to grow exponentially, contributing to a sevenfold increase in the global food supply during the 20th century.
 Environmental Impact & Concerns
Environmental Impact & Concerns
- While the Haber-Bosch process has been instrumental in solving global food shortages, it also presents significant environmental challenges.
- Excessive Nitrogen Use: The widespread use of nitrogen fertilizers has led to the accumulation of reactive nitrogen in the environment, which can cause soil degradation, water pollution, and air contamination. Excess nitrogen seeps into waterways through runoff, leading to problems like eutrophication—the excessive growth of algae that depletes oxygen in water bodies, killing aquatic life.
- Nitrogen Pollution: Reactive nitrogen released into the atmosphere can combine with water vapor, leading to acid rain, which damages ecosystems and erodes infrastructure.
- Greenhouse Gas Emissions: The production of ammonia through the Haber-Bosch process requires substantial amounts of energy, primarily from fossil fuels, contributing to the emission of greenhouse gases like carbon dioxide (CO2).
Global Food Security and the Haber-Bosch Process
- Despite its environmental drawbacks, the Haber-Bosch process remains essential for global food security.
- It is estimated that without synthetic fertilizers, about one-third of the world’s population—nearly 2 billion people—would face food shortages.
- The availability of nitrogen fertilizers has enabled countries to increase crop yields and sustain larger populations.
- For instance, in the early 20th century, India had an average life expectancy of only 19 years.
- By enabling the mass production of fertilizers, the Haber-Bosch process helped boost food production, contributing to improved health outcomes and increasing life expectancy to over 67 years today.
Technological Innovation and the Future
- While the Haber-Bosch process has been a crucial technological breakthrough, it is clear that technological solutions alone cannot solve the challenges of feeding a growing population.
- Experts suggest that along with continued innovation in fertilizer production, addressing these challenges requires political action, sustainable farming practices, and social mobilization to minimize environmental damage and ensure equitable food distribution.
- The lessons from the Haber-Bosch process underscore the importance of balancing technological progress with environmental stewardship and social responsibility.
Conclusion
- The Haber-Bosch process revolutionized agriculture by providing an artificial method to produce nitrogen fertilizers, enabling the world to meet its growing demand for food.
- However, its environmental impacts cannot be ignored, as excess nitrogen contributes to pollution, soil degradation, and climate change.
- Moving forward, the challenge lies in balancing the benefits of this technology with sustainable practices and innovation to ensure a healthier and more equitable future for all.









 Latest News
Latest News
 General Studies
General Studies