- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Hydrogen Fuel Cells
Hydrogen fuel cells, a type of Fuel Cells, offer immense promise as sources of clean energy for the future. These generate electricity by combining hydrogen (as a fuel) and oxygen electrochemically, producing only water and heat as byproducts. Hydrogen fuel cells are much quieter than gasoline or diesel vehicles and can be easily scaled up by increasing the fuel supply, unlike batteries, which require heavier components.
With their high efficiency, reliability, and zero emissions, hydrogen fuel cells can accelerate India’s move to renewable energy, energy security, and a reduction in oil imports, aligning with the UN Sustainable Development Goals.
Working of Hydrogen Fuel Cells
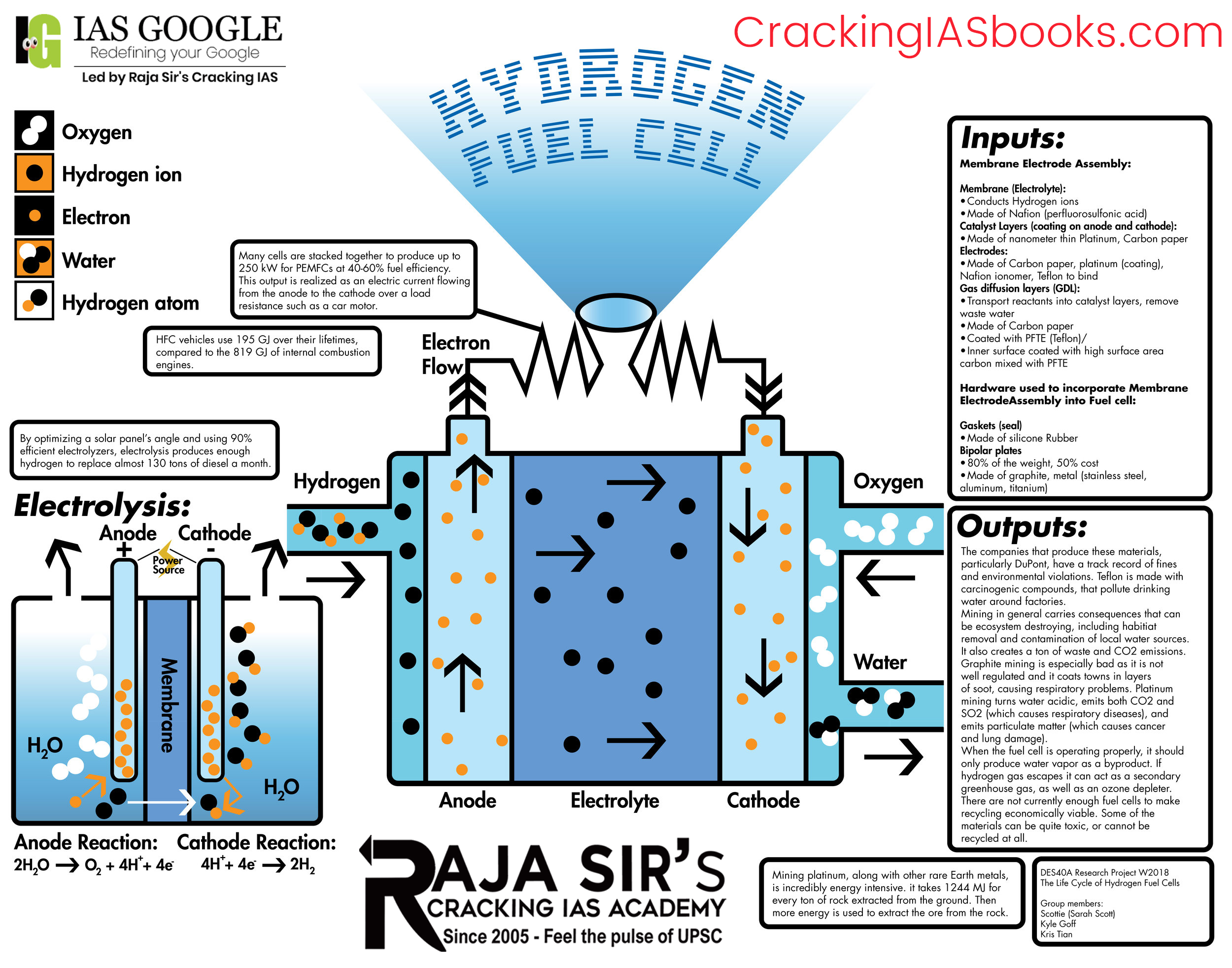
A hydrogen fuel cell converts the chemical energy stored in hydrogen into electrical energy via an electrochemical reaction with oxygen.
- Hydrogen fuel (H2) enters the fuel cell at the anode side and oxygen (O2) enters at the cathode side.
- At the anode, the hydrogen molecules come into contact with a platinum catalyst, which causes them to split into protons (H+) and electrons (e-). This process is called oxidation.
|
H2 → 2H+ + 2e- |
- The protons can pass through the Proton exchange membrane (example-Naflon), while the electrons travel along an external circuit creating current.
- At the cathode, the oxygen molecules interact with the platinum catalyst and split into oxygen atoms with a negative charge. This process is called reduction.
|
O2 + 4e- → 2O2- |
- The oxygen atoms then combine with the protons passed through the electrolyte and the electrons from the external circuit to produce water.
|
2H+ + 2e- + O2 → H2O |
- The reactions happen continuously, generating power as long as fuel and oxidants are supplied. The only byproducts are water and heat.
- The voltage generated by a single cell is about 0.7 V. To increase the voltage, many such cells are stacked together in a fuel cell stack.
In essence, hydrogen and oxygen gases are continuously fed to produce electricity sustainably, as long as fuel is supplied. The modular architecture also allows easy scaling of power capacity.
Methods for Producing Hydrogen Fuel
There are three main methods of producing hydrogen fuel:
- Steam Methane Reforming (SMR) - Hydrogen is produced by reacting natural gas with high-temperature steam. This is the most common production method currently, contributing around 95% of global hydrogen. However, it uses fossil fuels and emits carbon dioxide.
- Electrolysis - Water is split into hydrogen and oxygen by passing an electrical current. This allows the production of very pure hydrogen. Electrolyzers can be coupled with renewable electricity to generate ''green'' hydrogen sustainably.
- Thermochemical Processes - High-temperature heat is used to extract hydrogen from water and hydrocarbons. Thermochemical cycles require multiple chemical reactions, often using heat from nuclear plants or solar concentrators.
- In addition, hydrogen can be produced as a byproduct of industries like oil refining and chlorine production.
- Research is also aimed at developing biological and photobiological processes for clean hydrogen generation.
- Currently, steam reforming of methane dominates, but bringing down the costs of water electrolysis powered by renewables can make green hydrogen viable on a large scale.
Applications of Hydrogen Fuel Cells
Hydrogen fuel cells have diverse applications, from vehicles to electronics to backup power. Some major current examples are:
Electric vehicles:
- Fuel cell electric vehicles (FCEVs) powered by hydrogen have a much higher range and faster refuelling than battery EVs.
- FCEVs combine gasoline cars'' range and refuelling convenience with the environmental benefits of battery EVs.
- Leading auto manufacturers have demonstrated FCEV models and hydrogen refuelling infrastructures are expanding in several regions.
- Recently, Indian Railways unveiled a hydrogen fuel cell train with a top speed of 160 kmph and a range of 600 km per fill.
Stationary power:
- Stationary fuel cell systems can efficiently provide clean backup, off-grid and supplemental power for facilities, telecom towers, data centres etc.
- Large multi-megawatt installations can also feed power to electric grids. Their low-noise operation makes fuel cells suitable for hospitals, schools and urban areas.
- Bloom Energy installed fuel cells at a Tata Power data centre in India, producing over 1 MW of reliable clean energy.
- Apple uses fuel cells at its data center in North Carolina made by Bloom Energy.
Portable power:
- Small fuel cell systems can power portable electronic devices like smartphones, laptops and tablets for days without recharging.
- Fuel cartridge replacement provides instant recharging. Their miniaturisation, quiet operation and low heat make them ideal embedded power sources.
- UPS and DFA Aviation provide small fuel cells for military applications to power soldier equipment and unmanned aerial vehicles.
Benefits of Hydrogen Fuel Cells
Some key advantages driving research and adoption of hydrogen fuel cell technology are:
- Clean energy: Hydrogen Fuel cells have water vapour only as byproducts, hence are clean energy devices.
- High efficiency: Fuel cells can achieve electrical efficiency exceeding 60%, much higher than heat engines like internal combustion engines which have theoretical limits of around 25-30%.
- Reliable backup power: Fuel cell systems offer highly reliable and resilient backup power due to modular architecture. Easy hydrogen storage and instant refuelling provide an advantage over batteries.
Challenges in Adoption of Hydrogen Fuel Cells
Despite their benefits, certain key barriers need addressing for mass adoption of fuel cells:
- Cost barriers: Fuel cells currently have high costs due to their specialised materials like platinum catalysts.
- Mass manufacturing can reduce costs in future.
- Hydrogen storage issues: Effective, safe and economical hydrogen storage, especially onboard vehicles, remains an active research area.
- Options like high-pressure tanks, liquid hydrogen and metal hydrides are attractive alternatives.
- Lack of distribution in infrastructure: A vast network of pipelines, carriers and refuelling stations is needed for widespread fuel cell adoption, especially in transportation.
India’s Initiatives on Hydrogen Fuel Cells
Both government and private sector efforts are underway to build capabilities and infrastructure for hydrogen-based transport and energy.
- Green hydrogen clusters with integrated renewable capacity are being developed across India.
- R&D is being focused on electrolyzers, hydrogen storage materials, fuel cells and applications.
- Tata Motors and Ashok Leyland have developed hydrogen fuel cell buses demonstrated under the FAME scheme.
- Indian Railways is experimenting with hydrogen-powered trains and locomotives.
- Research institutions like IITs along with CSIR labs are active in fuel cell R&D and technology development.









 Latest News
Latest News
 General Studies
General Studies