- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Mitochondrial donation
Mitochondria are often referred to as the “powerhouses” of cells, as they generate energy in the form of adenosine triphosphate (ATP). Unlike nuclear DNA, which is inherited from both parents, mtDNA is exclusively passed down from mothers to their children. This means that any mutations present in a mother’s mitochondria can be transmitted to her offspring, potentially leading to severe mitochondrial diseases. These diseases can affect various systems in the body, including muscular, neurological, and metabolic functions.
- A group of diseases that affect mitochondria''s capacity to produce the energy organs need to function properly is known as mitochondrial disease (or Mito). Mito can have many different forms, each of which has the potential to disrupt one or more organs and lead to organ failure.
- Mito cannot be cured. However, a new IVF procedure known as mitochondrial donation gives families affected by certain forms of mito hope that they can have children who are genetically related to them without mito. Scientists are currently preparing for a clinical trial to determine whether mitochondrial donation is safe and effective, following the passage of a law in Australia in 2022 that will allow for the practice.
What is mitochondrial disease?
- There are two kinds of mitochondrial disease. One is brought by faulty genes in the nuclear DNA, which is what makes us who we are and is passed down from one parent to the other.
- The other is brought on by faulty genes in the DNA of the mitochondria. Mito caused by faulty mitochondrial DNA is passed down through the mother. However, the risk of disease is erratic, so a mother who is only mildly affected may give birth to a child with severe symptoms.
What are the symptoms of mitochondrial disease?
- One in every 5,000 people has mitochondrial disease, the most common inherited metabolic condition. While some individuals experience severe symptoms that progress rapidly, others have mild symptoms that progress slowly. Any organ can be affected by Mito, but the heart, brain, and muscles, which require a lot of energy, are more frequently affected than other organs.
- Mito that appears in childhood frequently includes different organs, progresses quickly, and has unfortunate results. About 60 of Australian babies born each year will develop mitochondrial disease, which can lead to death.
What is mitochondrial donation?
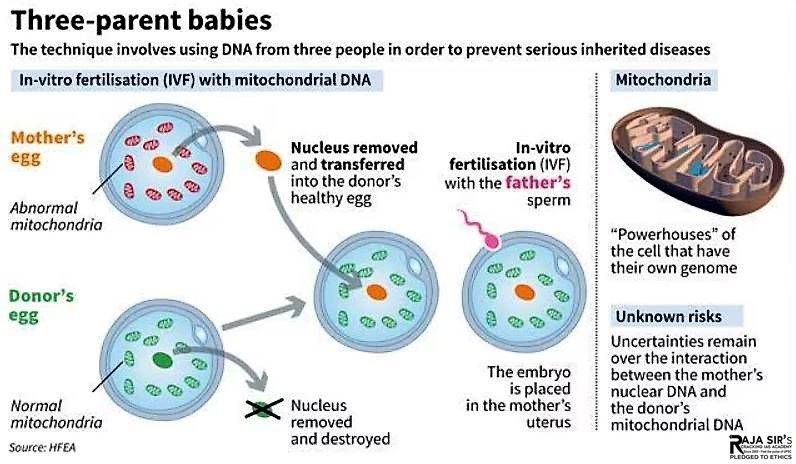
- Mitochondrial donation is a new IVF-based method that gives people with faulty mitochondrial DNA the chance to have children who are genetically related to them without passing on the faulty DNA. It involves removing the nuclear DNA from an egg donated by a person carrying faulty mitochondrial DNA and inserting it into an egg donated by someone who does not carry faulty mitochondrial DNA.
- The egg thus formed has the nuclear DNA of the intending parent and functioning mitochondria from the donor. After that, sperm are added, which makes it possible for the child to inherit the nuclear DNA of both intended parents. A child after mitochondrial donation will have genetic material from the three parties including nuclear DNA from the intending parents and mitochondrial DNA from the egg donor. As a result, the child will have less probability of getting mito or no probability at all.
What is the technical procedure of mitochondrial donation?
- Specially trained scientists and sophisticated equipment are required for this highly technical procedure. In addition, hormone injections are required for both the egg donor and the person with mito to stimulate the ovaries to produce multiple eggs.
- After that, an ultrasound-guided surgical procedure is used to retrieve the eggs. The United Kingdom is where mitochondrial donation first started, and as a result, a few babies have been born there. They have not been identified as being free of mito as of yet.
|
Mitochondrial Replacement Therapy (MRT) To prevent the transmission of mitochondrial diseases, scientists developed Mitochondrial Replacement Therapy (MRT), which involves replacing defective mitochondria in a mother’s egg with healthy mitochondria from a donor. There are two primary techniques used in MRT:
|
 What is the Maeve’s Law of mitochondrial donation?
What is the Maeve’s Law of mitochondrial donation?
- The Mitochondrial Donation Law Reform Bill 2021 was approved by the Australian Senate in 2022 after three years of public consultation. This makes mitochondrial donation legal for research and clinical trials. Maeve''s regulation specifies severe circumstances including that facilities need a unique permit to perform mitochondrial donation.
- The law also stipulates that initial licences will be issued for pre-clinical and clinical trial research and training in order to guarantee the safety and effectiveness of mitochondrial donation prior to its introduction into Australian clinical practice. We anticipate receiving one of these licences so that the mitoHOPE program, of which we are a part, can refine the method and carry out a clinical trial to ensure that mitochondrial donation is safe and effective.
- Embryologists will be trained in "real-life" clinical conditions and existing mitochondrial donation techniques will be refined and improved through a preclinical research and training program prior to the trial''s beginning. Numerous human eggs are required for this.
What is the need for the donor eggs?
- Finding eggs is one of the difficulties associated with mitochondrial donation. Frozen eggs can be used for the preclinical research and training program, but "fresh" eggs will be needed for the clinical trial. People who store eggs they don''t intend to use are one potential source of frozen eggs.
- In order to provide individuals affected by mito to have a chance at having a healthy baby, individuals will be required to volunteer to have their ovaries stimulated and eggs retrieved for the "fresh" eggs required in the future clinical trial. Egg donors can be people who know people who are participating in the trial or who don''t know anyone who has mito but would like to help them conceive.
- The goal is to begin enrolling participants in the clinical trial within the next 12 to 18 months at this stage. However, this could alter based on the timing of the licences and ethics approvals that are required.
What does the recent study show about stored eggs?
- Data on the outcomes of eggs stored at a Melbourne clinic from 2012 to 2021 were the subject of a recent study. 1,132 eggs from 128 patients were discarded over the ten-year period. Because the clinics where the eggs were stored did not conduct research that required donor eggs, no eggs were donated to research.
- However, research reveals that the most popular option for individuals who have stored eggs is to donate the eggs to research. This gives people hope that people who have eggs they don''t plan to use might be willing to donate them to mitochondrial donation preclinical research if given the chance.
Examples
- The First Three-Parent Baby: In 2016, a baby boy was born to Jordanian parents using MRT techniques developed by Dr. John Zhang at New Hope Fertility Center in New York City. The mother carried genes for Leigh syndrome, a severe neurological disorder caused by mitochondrial mutations that had previously led to the deaths of her first two children. The procedure was performed in Mexico due to regulatory restrictions in the U.S., and it successfully resulted in a healthy baby who shows no signs of disease.
- Subsequent Births: Following this groundbreaking birth, reports emerged of additional babies born using similar techniques in other countries. For instance, medical scientists in Ukraine reported births resulting from MRT applications aimed at treating infertility and preventing genetic diseases.
- UK Clinical Trials: In 2017, clinical trials began at Newcastle Fertility Centre in the UK after legislation was passed allowing MRT for preventing mitochondrial diseases. Although specific births resulting from these trials have not been widely publicized yet, they represent an important step toward regulated applications of this technology.
Concerns
The introduction of three-parent babies raises numerous ethical questions and safety concerns:
- Ethical Dilemmas: Critics argue that manipulating human embryos raises moral questions about “playing God” and could lead to unintended consequences in future generations. There are concerns about consent from donors and potential psychological impacts on children born through these methods.
- Health Risks: Long-term health effects of MRT on children born through these techniques remain largely unknown. While initial results may be promising, there is concern about potential complications arising from having genetic material from three different individuals.
- Regulatory Challenges: Different countries have varying regulations regarding MRT and genetic manipulation technologies. In some places, such as the United States, strict regulations limit research and application of these techniques, while others like the UK have embraced them under controlled conditions.
- Designer Babies: There is apprehension that advancements in genetic manipulation could lead to “designer babies,” where parents might select traits or characteristics beyond health considerations.
Conclusion
- Three-parent babies represent a significant advancement in reproductive technology aimed at preventing mitochondrial diseases while enabling families affected by these conditions to have healthy children. Through techniques like Mitochondrial Replacement Therapy (MRT), scientists are exploring new frontiers in genetics that hold promise for treating hereditary diseases.
- However, as with any groundbreaking technology, ethical considerations and safety concerns must be carefully addressed through rigorous scientific research and regulatory oversight. Ongoing studies will help determine long-term outcomes for children born via these methods while ensuring that this technology is used responsibly and ethically.
- As our understanding of genetics continues to evolve alongside technological advancements, three-parent babies may pave the way for new possibilities in reproductive health and disease prevention—offering hope to families affected by devastating genetic disorders while challenging us to consider the moral implications of such innovations.









 Latest News
Latest News
 General Studies
General Studies