- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
 EDITORIALS & ARTICLES
EDITORIALS & ARTICLES
GEMCOVAC-19, India's first mRNA Covid-19 vaccine, scores over Pfizer, Moderna
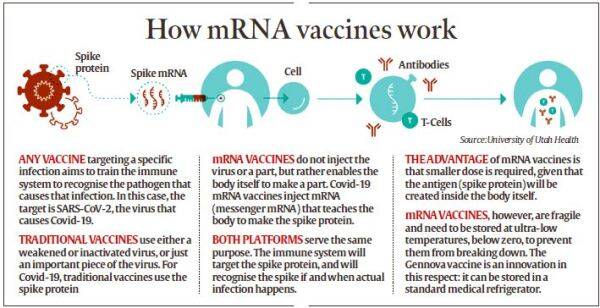
The country’s first home-grown mRNA Covid-19 vaccine — GEMCOVAC-19 — developed at Pune’s Gennova Biopharmaceuticals has got a ‘restricted emergency use’ nod for the 18-and-above age group. As mRNA vaccines are required to be kept at sub-zero temperatures, it was a mammoth task for Gennova scientists to develop a thermostable mRNA Covid-19 vaccine. Scientists had to innovate to suit local needs to make it affordable and deployable. The new vaccine can now be stored at the temperature of a standard medical refrigerator.
The mRNA platform
As the Covid-19 pandemic spread, an mRNA vaccine candidate was the first to enter human trials globally. The first two vaccines that were made available for use in the US were based on mRNA technology.
Unlike vaccines that put a weakened or inactivated virus in your body to activate an immune response, these two Covid-19 vaccines (Pfizer-BioNTech and Moderna) used messenger RNA or mRNA to deliver a message to your immune system.
Basically, the technology uses genetically engineered mRNA to instruct cells to make the S-protein found on the surface of the Covid-19 virus. According to reports from US-based Mayo Clinic, after vaccination, the muscle cells begin making S-protein pieces and displaying them on cell surfaces. This causes the body to create antibodies.
But these vaccines have to be stored at sub-zero temperatures as mRNA is fragile and breaks down easily.
Thermostable vaccine
“Unlike in the West, where the vaccine has to be stored at sub-zero temperatures, the challenge in India was to be able to store the vaccine between 2-8 degree Celsius. We had to innovate to suit our local needs as to what is affordable and deployable. GEMCOVAC-19 can now be stored at the temperature of a standard medical refrigerator,” says Dr Sanjay Singh, CEO of Gennova Biopharmaceuticals.
The conversion from liquid to powder form of the vaccine takes place via Lyophilisation — this is freeze-drying, a process where the water is removed from the product after it is frozen and placed under a vacuum allowing the ice to change directly from solid to vapor without passing through a liquid phase.
However, just removing water by Lyophilisation of the mRNA vaccine does not work. So, the surrounding pressure has to be tweaked and then kept stable to ensure the characteristics of the vaccine are the same as before Lyophilisation. For this to be achieved, the key was to add an external agent which at a certain critical concentration keeps it stable under lyophilized conditions. The Lyophilisation technology is not new, but a lyophilized mRNA vaccine is unique.
“We performed hundreds of trials before arriving at the right formulation and right condition to ensure a heat-stable mRNA vaccine,” Dr Singh said.
Trials and safety
Freeze-drying the large and unstable mRNA molecule with the nanoparticle was a daunting challenge. However, Gennova invested countless man-hours in the hope of lyophilizing the mRNA vaccine in a single vial within a year. This thermostable vaccine was thoroughly tested in various animal models to ensure its safety and immunogenicity before entering human clinical trials. Phase 1 and 2 trial data across 480 participants had been submitted earlier, and data from Phase 3 trial across 4,000 participants was then presented to the Central Drugs Standard Control Organisation (CDSCO). During the Phase 3 trials, 3,000 participants were administered the mRNA Covid-19 vaccine and 1,000 were given Covishield.
According to officials at Gennova, the trial data showed that the vaccine was safe and well-tolerated. Immunogenicity measured at 2 weeks post-dose showed that GEMCOVAC-19 is non-inferior to Covishield.
The two-dose vaccine will have to be administered intramuscularly, 28 days apart.
Fight against emerging variants
For the first time, the mRNA platform has been used to develop a Covid-19 vaccine in India. This total process may have taken one-and-a-half years, but for Dr. Singh, a biochemist who had worked on malaria vaccines at the US-based National Institutes of Health, and the team, designing an mRNA vaccine against the Omicron variant barely took 60 days.
Notably, this technology platform provides flexibility to quickly tweak the vaccine for any existing or emerging variants of the virus.
“A pandemic-ready platform technology has been created that can be used to quickly develop a vaccine should a variant-of-concern emerge due to the rapid mutation of the SARS-CoV-2 virus. Clinical trials need to be done to ascertain the effectiveness of the GEMCOVAC-19 against Omicron and sub-variants,” said Dr. Singh.
A short clinical trial will also be conducted with the Omicron-specific vaccine, which has also been designed for use as a booster and a protocol submitted to the DCGI.
He added: “The learning curve was steep not only in terms of production but also in conducting the clinical trials. The approval of the nation’s first mRNA vaccine will pave the way for the development of new-variant specific mRNA vaccines that can be used as future booster doses. It was absolutely necessary to establish the safety and immunogenicity of the new vaccine platform technology in the Indian population.”
Ready for roll-out
Gennova already has a license to manufacture and sell from the CDSCO.
Gennova Biopharmaceuticals Chief Operating Officer Samit Mehta said that talks are underway with the government on whether they would like to procure and deploy or whether the firm can reach out to the private market. On pricing, he added: “Compared to our peers we will be competitive.”
Authorities at Gennova said that they are actively engaged in talks with at least 25-30 countries that had evinced interest in the new vaccine.









 Latest News
Latest News General Studies
General Studies