- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Mitochondrial Replacement Techniques - PPP 100 - PRELIMS 2024 - 8
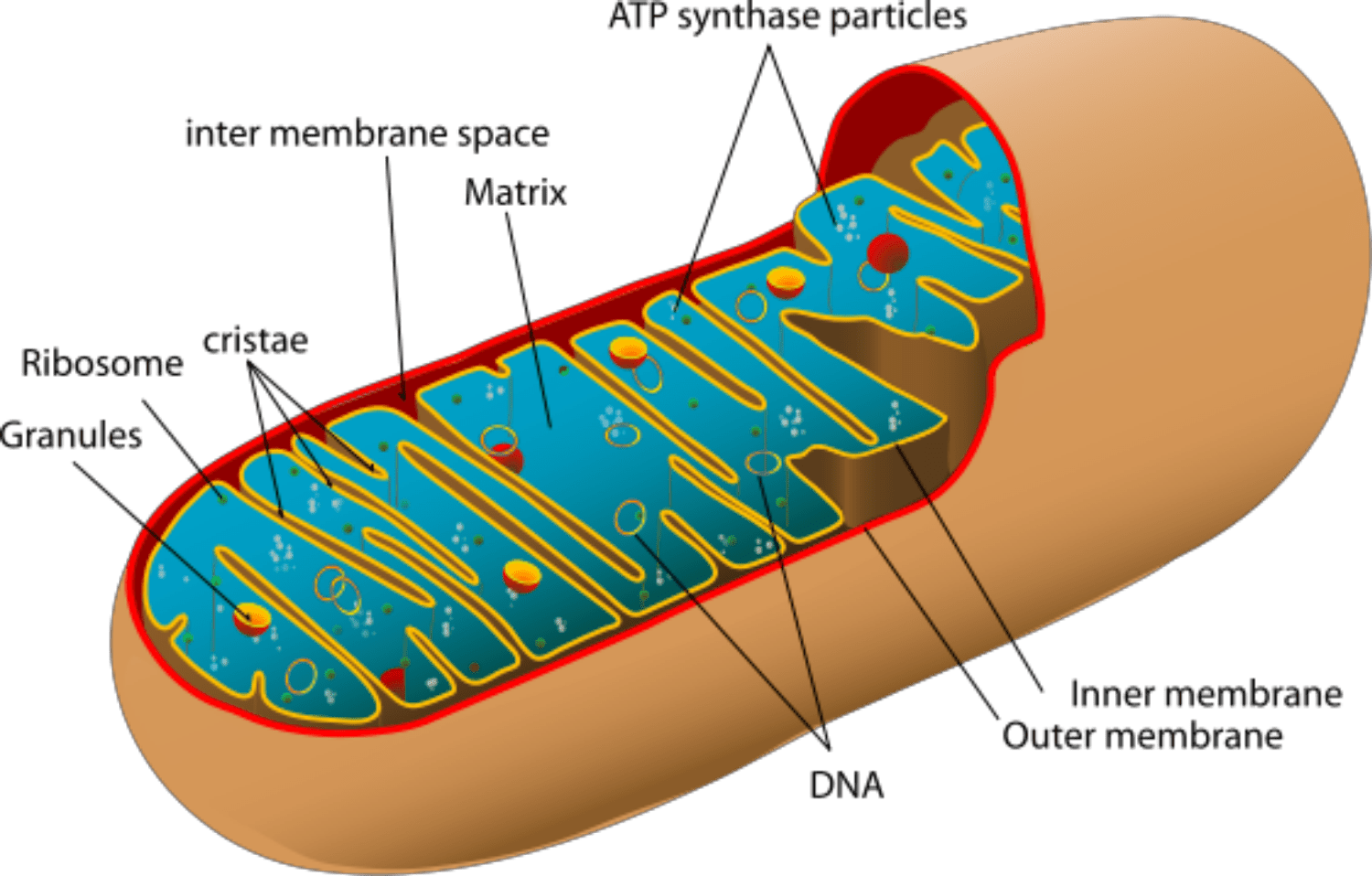
Mitochondria are organelles that are a part of almost every cell in the human body. Their function is to ensure the proper performance of the cell by supplying it with energy that we get, like with the food we eat. In case of mitochondrial failure, our cells do not receive the power to operate properly, which leads to damage in bigger systems, like organs.
Mitochondria are present in the eggs, too — they help them to work right to be suitable for fertilization and then grow into a healthy fetus. If there is any type of mitochondrial damage in the egg, the fetus is at risk of developing mitochondrial diseases. To prevent mitochondrial disease with donations, donor eggs are used to get a healthy environment for the genetic material of the parents.
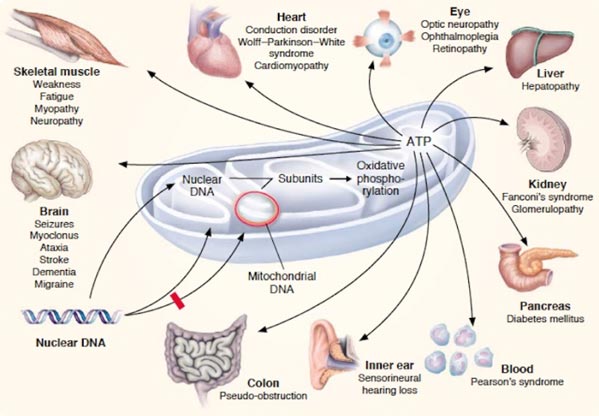
Mitochondrial disease
Mitochondrial diseases belong to severe medical disorders that can be inherited, meaning that the transmission of mitochondrial DNA (mtDNA) from the mother to the child is quite likely. In the case of mitochondrial disease, genetic mutations take place and lead to the inability of mitochondria to derive energy from oxygen and food.
In other words, the risk of passing the disease from the mother to the child translates to the risk of the child''s organelles being unable to perform mitochondrial functions. This causes cell damage and even death.
The presence of mutated mtDNA in the body leads to illnesses and, therefore, poor quality of life. And in the case of severe mitochondrial disease, one may go through organ and system failures, eventually dying at a young age.
One of the ways to treat mitochondrial diseases is with genetic research and the donation of biomaterials.
Mitochondrial donation
Mitochondrial donation is a type of assisted reproductive technology performed by a fertilization and embryology authority to prevent mitochondrial disease in the fetus. It is performed as a part of the in vitro fertilization (IVF) cycle. Most often, it is recommended to introduce mitochondrial DNA donation to couples where mothers have the disease and, therefore, pose a risk of passing them to the child.
Mitochondrial donation techniques imply that a healthy woman donates an egg for the couple going through IVF to use for conception. The fertilization and embryology experts then perform a procedure of removing the mitochondrial DNA mutations from the egg that will be placed in the patient’s uterus to prevent the transmission of the disease.
In such a way, the woman gets a healthy egg with unhealthy mitochondria removed and fertilized with mitochondrial genes from the donated egg. This is the method used to avoid mitochondrial disease transmission from the mother to the child with assisted reproductive technology.
Mitochondrial Replacement Techniques
MRT is an in vitro fertilization (IVF) technique that involves removing an intended mother''s nDNA from her oocyte or zygote, which contains mutated mtDNA, and transferring it into a female provider''s oocyte or zygote, which contains nonpathogenic mtDNA and from which the nDNA has been removed.2 The woman providing oocytes would have no personal or family history or genetic evidence of having mutated, pathogenic mtDNA. In this report, the term “MRT” encompasses both the transfer of the nuclear genetic material and the accompanying fertilization procedure that is necessary to produce a human embryo. These techniques could allow intended mothers to produce a child that would share their nDNA without passing on their pathogenic mtDNA. Three such techniques are most advanced in development: maternal spindle transfer (MST); pronuclear transfer (PNT); and, most recent, polar body transfer (PBT).
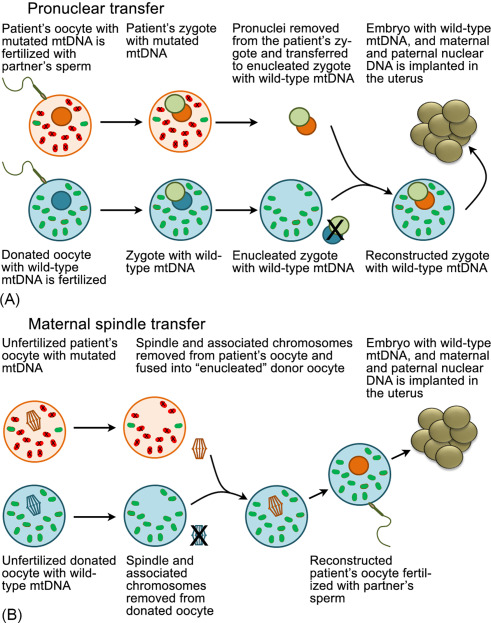
A. Maternal Spindle Transfer (MST)
In this technique, the nuclear chromosomes, which are grouped in a spindle formation, would be removed from both an oocyte provided by a woman with nonpathogenic mtDNA and the intended mother''s oocyte. The intended mother''s oocyte, containing mutated mtDNA, would be discarded. The intended mother''s nuclear chromosomes would be inserted into the provided oocyte, which would contain nonpathogenic mtDNA. The oocyte would then be fertilized with the intended father''s or another man''s sperm. Following fertilization, the embryo would be grown in culture and subjected to diagnostic testing to ensure its quality and viability; the testing would include preimplantation genetic diagnosis (PGD) to confirm that the embryo had acceptably low or undetectable levels of the pathogenic mtDNA molecules. The resulting embryo(s) would be frozen until test results confirmed suitability for transfer and then transferred into the uterus of the intended mother (or gestational carrier, if needed).
B. Pronuclear Transfer (PNT)
In this technique, both an oocyte provided by a woman with nonpathogenic mtDNA and the intended mother''s oocyte would be fertilized with sperm in vitro, creating two zygotes. The maternal and paternal pronuclei, which contained the nDNA, would be removed from both zygotes. The intended mother''s enucleated zygote, containing pathogenic mtDNA, would be discarded. The pronuclei from the intended mother''s zygote would be inserted into the enucleated zygote created with the provided oocyte and the intended father''s (or another man''s) sperm, which would contain nonpathogenic mtDNA. The resulting embryo(s) would then be grown, tested, and transferred as detailed above for MST.
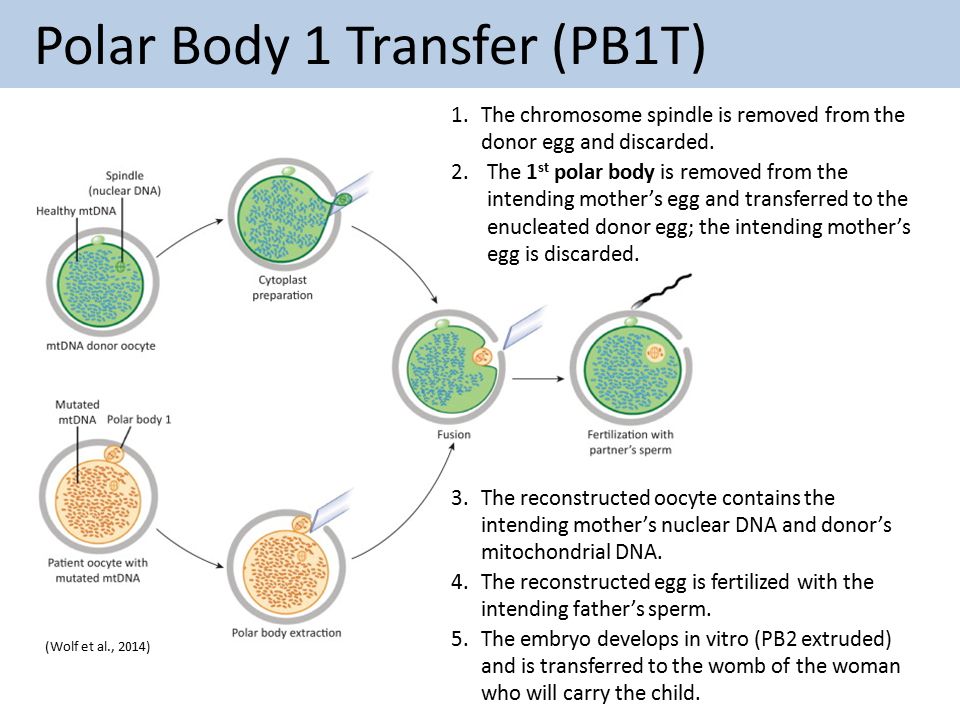
C. Polar Body Transfer (PBT)
There are two versions of PBT. In polar body 1 transfer (PB1T), the intended mother''s first polar body, which is a by-product of oogenesis, containing her nDNA and very little mtDNA, would be transferred to an oocyte provided by a woman with nonpathogenic mtDNA from which the nDNA had been removed. The reconstructed oocyte would then be fertilized, grown, tested, and transferred as detailed above for MST. In polar body 2 transfer (PB2T), both the intended mother''s oocyte and an oocyte provided by a woman with nonpathogenic mtDNA would be fertilized. The intended mother''s second polar body, containing nDNA and very little mtDNA, would be transferred to the zygote of the woman who provided the oocyte, from which the pronuclei had been removed. The resulting embryo(s) would then be grown, tested, and transferred as detailed above for MST.
D. Germinal Vesicle Transfer (GVT)
The germinal vesicle is the large nucleus present in immature (primary) oocytes. Primary oocytes remain arrested in prophase 1, with the nucleus as germinal vesicle within the ovary, for years until they are stimulated in successive menstrual cycles, after puberty. GVT consists in transplanting the germinal vesicle from a patient’s primary oocyte to a donor oocyte in which the germinal vesicle has also been extracted. Since these are immature oocytes, the resulting transplanted oocyte will be subjected to a maturation process in vitro to complete the meiosis. After that, it will be fertilized and implanted.
Issues with mitochondrial donation
Since mitochondrial research on donation is relatively recent and continues, there are still some uncertainties associated with it. There 3 main areas of concern are ethical, social and legal.
Ethical issues
- One of the ethical concerns regarding assisted reproductive technology methods to avoid mitochondrial disease transmission implies that mitochondrial replacement therapy involves working with human embryos. Since they are considered morally significant, the changes in the mutated mitochondrial DNA performed on the embryos are, therefore, a major concern in the debate.
- Another ethical issue related to the donation of mitochondrial material is that humans get involved in the natural processes. While the long-term goal is to improve national health, the fact that there''s human interference and genetic modification makes it an ambiguous practice in terms of ethics.
Social issues
- One of the biggest social issues related to introducing mitochondrial donation techniques is the effects it may have on humanity as a whole. Since the history of mitochondrial donation is not that long, the consequences of mitochondrial transfer on future generations are unknown in terms of people’s physical health and relationships, as well as changes in their genetic material.
- Another concern is caused by the fact that a third person is involved in the process and implies the ambiguity of their role in the future family. The way things will stand will be uncertain if the child is eager to start a relationship with the person who participated in the donation of mitochondrial DNA.
- Lastly, one more social concern is that gene therapy is not accessible to everyone — only social groups that are economically forward can use it. The cost of the practice and access to medicine, in general, is a privilege that not all individuals have.
Legal issues
- As for the legal issues that are associated with the prevention of mitochondrial diseases, these are also related to the use of human biomaterial and the involvement of the people who donate mitochondrial DNA. For example, the issue with using human eggs for donation implies that egg trade may occur, which poses a risk to the rights, freedom and safety of people.
- At the same time, the problem with involving a third person creates an ambiguous situation in terms of the right the person gains on the child and the child''s right to reach out to the donor. Even if the donor agrees to have no interest in the child any time in the future, the issue of information retention comes up because the donor’s data still needs to be kept for scientific and medical reasons.
Risks of mitochondrial donation
- There are some risks that the methods of mitochondrial diseases transmission prevention pose. One of them is that there is no complete guarantee that it will help prevent the transmission of mitochondrial DNA disorders to the embryo. While the process requires extracting the mtDNA disease and placing it in the healthy egg, there are still chances that all unhealthy organelles are not eliminated from the embryo and enter the embryo.
- The reason is that some faulty organelles can still get stuck to the DNA in the course of the procedure, and there''s nothing fertilization and embryology authority representatives can do about it. With this being said, there is no 100% guarantee that the procedure will prevent the mitochondrial disease from transmission to the fetus.
- Then, the extent of mitochondrial DNA diseases will affect the success of the procedure, too. If the unhealthy organelles are still there when an embryo is created, the way they will be balanced with the healthy ones is unpredictable. Sometimes the healthy DNA will prevail, but in some cases, the disease will still be present in the DNA after the procedure and keep the disease active and evolving. This may lead to the occurrence of severe mitochondrial disease in the fetus or even the next generation.
- Lastly, there is a chance of a mismatch between the mitochondrial donor''s and mother''s DNA. Depending on the origins of the mitochondrial donor and the mother, there may appear a mismatch of the mitochondrial genome haplotypes. While this does not mean that the mitochondrial replacement techniques will be ineffective, there are still potential risks of mitochondrial donation caused by the mismatch.
Advantages of mitochondrial donation
- While there are certain risks associated with mitochondrial gene donation, the advantages of mitochondrial donation remain strong. In general, it is one of the most effective methods to avoid mitochondrial diseases in children in case of the mtDNA disease presence in the mother. That’s why patients should have mitochondrial DNA donation in case of being under threat of transmission.
- Because of the severe consequences of the disease on the person''s health and quality of life, the ability of fertilization and embryology specialists to prevent mitochondrial diseases is a significant opportunity. In case of a successful procedure, it is possible to eliminate the present type of mitochondrial diseases in the family and improve national health in the long run.
PYQs
1] In the context of hereditary diseases, consider the following statements: [2021]
1) Passing on mitochondrial diseases from parent to child can be prevented by mitochondrial replacement therapy either before or after in vitro fertilisation of the egg.
2) A child inherits mitochondrial diseases entirely from mother and not from father.
Which of the statements given above is/are correct?
(a) 1 only
(b) 2 only
(c) Both 1 and 2
(d) Neither 1 nor 2
2] In the context of recent advances of human reproductive technology, “Pronuclear Transfer” is used for: [2020]
(a) Fertilization of egg in vitro by the donor sperm
(b) Genetic modification of sperm producing cells
(c) Development of stem cells into functional embryos
(d) Prevention of mitochondrial diseases in offspring









 Latest News
Latest News
 General Studies
General Studies