- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
Discuss in detail the photochemical smog emphasizing its formation, effects and mitigation. Explain the 1999 Gothenburg protocol. (UPSC CSE Mains 2022 - General Studies Paper 3)
- Photochemical smog, also known as summer smog, is a type of smog that is produced when UV light originating from the sun interacts with the oxides of nitrogen present in the atmosphere. This type of smog usually manifests as a brown haze and is most commonly seen in highly populated cities that are placed in relatively warm climates. Furthermore, photochemical smog is most prominently visible during the mornings and afternoons.
Formation of Photochemical Smog
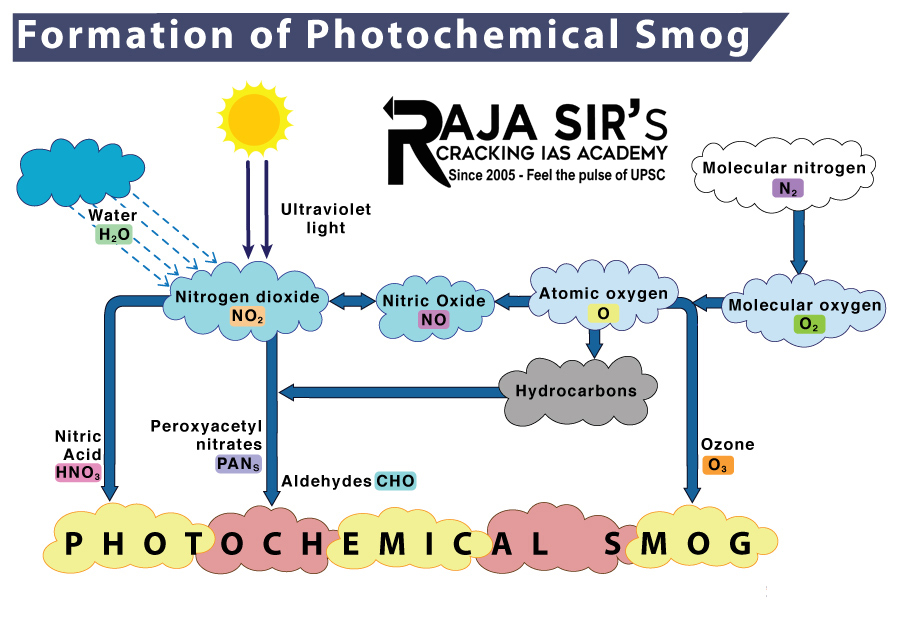
- Photochemical smog is formed by a complex series of chemical reactions involving sunlight, oxides of nitrogen, and volatile organic compounds that are present in the atmosphere as a result of air pollution. These reactions often result in the formation of ground level ozone and certain airborne particles. The formation of photochemical smog is closely related to the concentration of primary pollutants in the atmosphere. It is also related to the concentration of secondary pollutants (in some cases).
- Common examples of primary pollutants that contribute towards photochemical smog include oxides of nitrogen such as nitric oxide, nitrogen dioxide, and nitrous oxide and most VOCs (volatile organic compounds). Common examples of secondary pollutants that contribute towards the formation of photochemical smog include aldehydes, tropospheric ozone, and peroxyacyl nitrates (often abbreviated to PAN).
- During peak-traffic hours in the morning, large amounts of nitrogen oxides and volatile hydrocarbons are released into the atmosphere. These pollutants can be traced to automobile emissions and industrial discharge. Some of these hydrocarbon pollutants rapidly undergo oxidation by the hydroxyl groups in the atmosphere, resulting in the formation of peroxy radicals. These peroxy radicals go on to convert nitric oxide into nitrogen dioxide.
|
ADDITIONAL INFO - NOT TO BE INCLUDED IN ANSWER Nitric oxide (NO) Nitric oxide (NO) is a gas formed by combining nitrogen and oxygen. It occurs naturally both outside and inside the body. Outside the body, nitric oxide is a colorless, sweet-smelling gas that is toxic at high levels. Inside the body, it acts as an important chemical messenger involved in many bodily functions. Nitric oxide can also become toxic inside the body when levels get too high. Research has shown nitric oxide plays a role in neurotransmission, or information sharing between neurons, which helps functions in the nervous system like digestion and memory. It may also encourage the release of hormones, including growth hormones and insulin. Nitric oxide also acts as a vasodilator, meaning it helps open blood vessels to improve blood flow. It may also improve immunity by promoting inflammation to fight infections. There are no supplements that contain nitric oxide directly. However, our body uses certain amino acids—L-arginine and L-citrulline—to produce nitric oxide, and these amino acids are available in supplement form. Taking these supplements can raise the amount of nitric oxide in our body. Another way to get the benefits of increased nitric oxide levels is to eat foods that contain nitrates, which are compounds made of nitrogen and oxygen. Nitrates are found primarily in water and leafy vegetables. Nitrous oxide Nitrous oxide, commonly known as laughing gas or happy gas, is a colorless, non-flammable gas, with a sweetish odor. It's used in medical and dental procedures as a sedative, relieving anxiety and allowing you to relax before any work is done. Nitrous oxide was first used as an anesthetic in the 1800s. Nowadays, it's usually combined with oxygen for sedation purposes and is the most-used gas anesthetic in the world. Outside of medicine, nitrous gas is used for aerosol whipped cream and cooking spray; in automobile racing to make engines to go faster; and as a recreational drug. Nitrous oxide is a depressant, so it slows our body down. Once it kicks in, you may feel: Happy Giggly Light-headed Mild euphoria Relaxed Nitrous oxide gets the name “laughing gas” because of these effects. Some people have mild hallucinations while under its influence. Volatile organic compounds (VOCs) Volatile organic compounds (VOCs) are emitted as gases from certain solids or liquids. VOCs include a variety of chemicals, some of which may have short- and long-term adverse health effects. Concentrations of many VOCs are consistently higher indoors (up to ten times higher) than outdoors. VOCs are emitted by a wide array of products numbering in the thousands. Organic chemicals are widely used as ingredients in household products. Paints, varnishes and wax all contain organic solvents, as do many cleaning, disinfecting, cosmetic, degreasing and hobby products. Fuels are made up of organic chemicals. All of these products can release organic compounds while you are using them, and, to some degree, when they are stored. Sources of VOCs - Household products, including: paints, paint strippers and other solvents wood preservatives aerosol sprays cleansers and disinfectants moth repellents and air fresheners stored fuels and automotive products hobby supplies dry-cleaned clothing pesticide Other products, including: building materials and furnishings office equipment such as copiers and printers, correction fluids and carbonless copy paper graphics and craft materials including glues and adhesives, permanent markers and photographic solutions. Nitrogen Dioxide (NO2) Nitrogen Dioxide (NO2) is one of a group of highly reactive gases known as oxides of nitrogen or nitrogen oxides (NOx). Other nitrogen oxides include nitrous acid and nitric acid. NO2 is used as the indicator for the larger group of nitrogen oxides. NO2 primarily gets in the air from the burning of fuel. NO2 forms from emissions from cars, trucks and buses, power plants, and off-road equipment. Effects of NO2 Health effects
Environmental effects
|
Effects of Photochemical smog
Health Effects
- Photochemical smog is capable of inflicting irreversible damage on the lungs and heart. Even short-term exposure to photochemical smog tends to have ill effects on both the young and the elderly. It causes painful irritation of the respiratory system, reduced lung function and difficulty breathing; this is more evident while exercising or working outdoors. High levels of smog also trigger asthma attacks because the smog causes increased sensitivity to allergens, which are triggers for asthma.
- People with pre-existing health problems (such as respiratory diseases) are sensitive to ozone. Children, the elderly and people with poor lung function carry a far greater risk of developing respiratory illness from photochemical smog than healthy adults.
Effects on Environment
- Photochemical smog has devastating effects on the environment. The collection of chemicals found in photochemical smog causes problems for plants and animal life. Some plants such as tobacco, tomato and spinach are highly responsive to ozone, so photochemical smog can decimate these sensitive crops, trees and other vegetation. Ozone causes necrotic (dead) patterns on the upper surfaces of the leaves of trees. Ground-level ozone also can interfere with the growth and productivity of trees. The effects of smog on animals are also similar to its effect on humans; it decreases lung capacity and lung elasticity.
Mitigation
- Take precautionary steps to safeguard against the ill effects of photochemical smog. Generally, photochemical smog is less concentrated in the early morning or evening; therefore, exercising and planning outdoor activities during this part of the day limits smog exposure. Emissions from cars and other vehicles are the largest sources of smog. Reduce your daily pollutant emissions by driving less, making use of carpools, and maintaining the car in good condition. Other small actions, such as tightly sealing the lids of chemical products like garden chemicals, solvents, and household cleaners, minimizes evaporation of the chemicals and helps reduce smog.
- The problem of photochemical smog has also prompted some more serious reforms in an effort to reduce emissions. Switching over to other types of fuels, desulfurization of fuel gases from coal-fired power plants, expansion of public rail transport and low emission application of fertilizer in agriculture are some of the steps which have drastically reduced the level of photochemical smog.
Gothenburg Protocol 1999 (Multi Effect Protocol)
- The official title of the protocol is UNECE Protocol to Abate Acidification, Eutrophication, and Ground-level Ozone (Gothenburg Protocol) (Protocol to the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP))
- The Gothenburg Protocol was established to address pollutants that cause acidification and ground-level ozone.
- It sets limits on air pollutants including sulfur dioxide, nitrogen oxide, ammonia, and volatile organic compounds that are hazardous to human health and the environment.
- It was updated in 2012 to include particulate matter (PM) and black carbon (as a component of PM) and to include new commitments for 2020.
- The Protocol establishes legally binding emissions reduction commitments for 2020 and beyond for the major air pollutants:
- sulfur dioxide (SO2), nitrogen oxides (NOx), ammonia (NH3), volatile organic compounds (VOCs), and delicate Particulate Matter (PM5).
- these chemicals also contribute to photochemical smog formation.
The objective of the Gothenburg Protocol under the United Nations Economic Commission for Europe’s (UNECE) LRTAP Convention is:
-
- to control and reduce emissions of sulfur dioxide (SO2), nitrogen oxides (NOx), ammonia (NH3), volatile organic compounds (VOCs), and PM that are caused by human activities.
- to ensure that atmospheric depositions or concentrations do not exceed critical loads/levels;
- that Parties give priority, to implementing measures to reduce PM, to sources that are also significant sources of black carbon to provide benefits for human health and the environment and to help mitigation of near-term climate change.
- This multi-pollutant, multi-effect protocol is meant to eventually replace the older protocols that cover the same pollutants.
- Thus, when all Parties ratify the amended Protocol, their obligations under the following existing protocols: SO2 (1985 Helsinki and 1994 Oslo Protocols); NOx (1988 Sofia Protocol); and VOC (1991 Geneva Protocol); will become null and void.
- India has not signed the protocol.









 Latest News
Latest News
 General Studies
General Studies