- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
 EDITORIALS & ARTICLES
EDITORIALS & ARTICLES
2019 Nobel Prizes in Science
The Nobel Prizes -- a set of awards honoring the best work in physics, literature, chemistry, medicine, peace and economics -- are viewed as the highest intellectual honor in the world. Alfred Nobel, peace advocate and inventor of dynamite, left the money for the first five awards in his will. In 1901, the Nobel prizes were born.
Physics
Our understanding of the origin and evolution of the universe has undergone many changes in the past 100 years since the Belgian astronomer Georges Lemaître proposed what became known as the Big Bang theory. Cosmic background radiation was discovered in 1965 and turned out to be a gold mine for our understanding of how the universe developed from its early childhood to its present day. On another scale, a major discovery of an Earth-like planet orbiting a sun-type star outside our solar system was made in 1995. Together these discoveries led to a new understanding of our place in the universe. 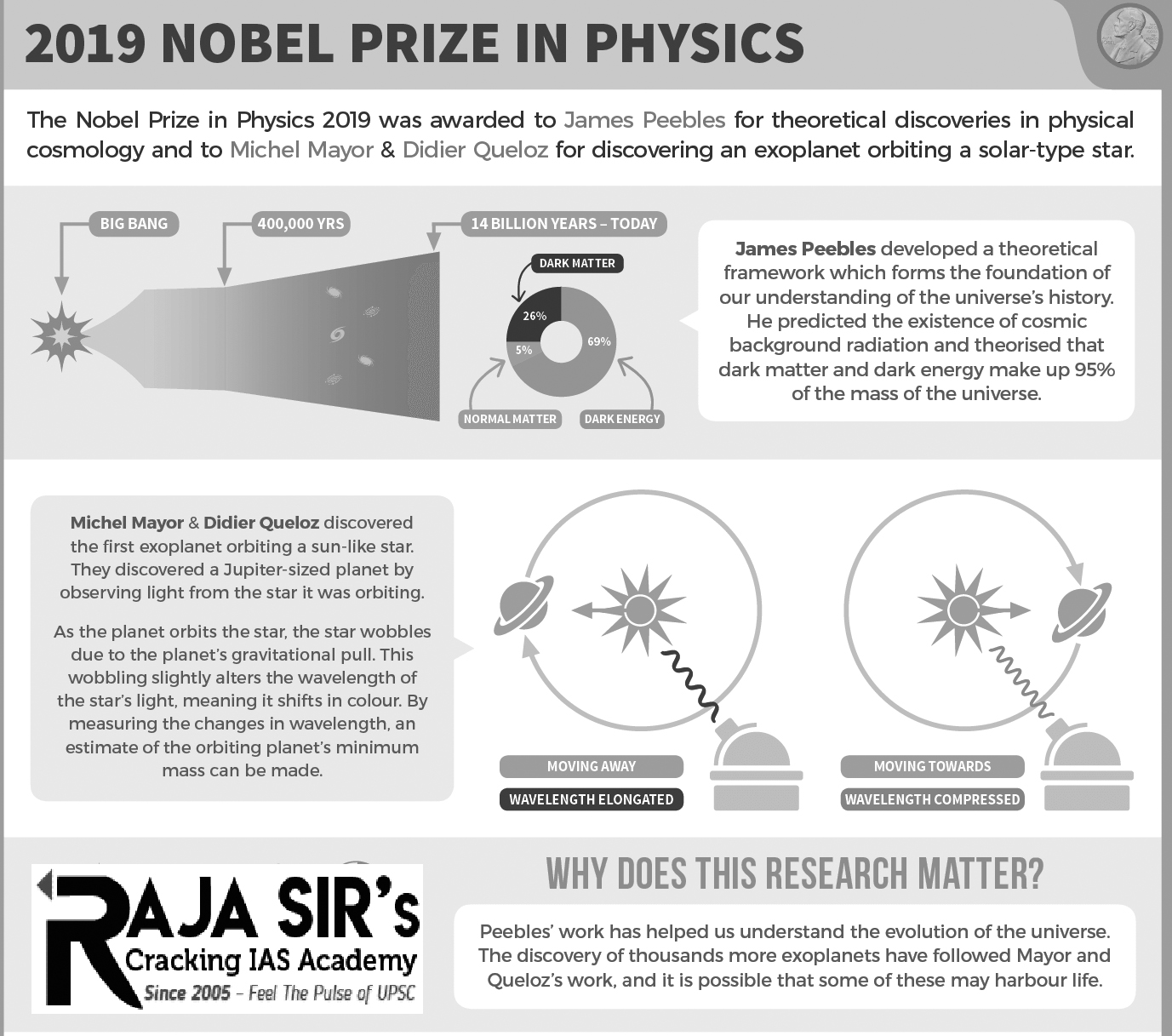
The 2019 Nobel Prize in Physics has been awarded to three scientists “for contribution to our understanding of the evolution of the universe and Earth’s place in the cosmos”. James Peebles of Princeton University, USA, receives the prize “for theoretical discoveries in physical cosmology” while Michel Mayor of the University of Geneva, Switzerland and Didier Queloz, of the University of Geneva and Cambridge University, UK, have been awarded “for the discovery of an exoplanet orbiting a solartype star”.
The Big Bang model describes the universe from its very first moments, almost 14 billion years ago, when it was extremely hot and dense. Since then, the universe has been expanding, becoming larger and colder. Barely 400,000 years after the Big Bang, the universe became transparent and light rays were able to travel through space. Using his theoretical tools and calculations, James Peebles was able to interpret these traces from the infancy of the universe and discover new physical processes.
The results showed us a universe in which just five per cent of its content is known, the matter which constitutes stars, planets, trees – and us. The rest, 95 per cent, is unknown dark matter and dark energy – that still remain a mystery and a challenge to modern physics. Peebles’ insights into physical cosmology have enriched the entire field of research and laid a foundation for the transformation of cosmology over the last fifty years, from speculation to science. His theoretical framework, developed since the mid-1960s, is the basis of our contemporary ideas about the universe.
In October 1995, Michel Mayor and Didier Queloz announced the first discovery of a planet outside our solar system, an exoplanet, orbiting a sun-type star in our home galaxy, the Milky Way. Before this finding, the only confirmed exoplanet known orbited a pulsar – a dense remnant from a supernova explosion. Using custommade instruments at the Haute-Provence Observatory in southern France, they were able to see planet 51 Pegasi b, a gaseous ball comparable with the solar system’s biggest gas giant, Jupiter. Mayor and Queloz carefully measured a star’s velocity using Doppler shift and found that it wobbles back and forth in a tell-tale pattern produced by the gravitational pull of an orbiting planet. This discovery started a revolution in astronomy and over 4,000 exoplanets have since been found in the Milky Way.
This year’s laureates have transformed our ideas about the cosmos. While James Peebles’ theoretical discoveries contributed to our understanding of how the universe evolved after the Big Bang, Michel Mayor and Didier Queloz explored our cosmic neighbourhoods on the hunt for unknown planets. Their discoveries have forever changed our conceptions of the world and strange new worlds are still being discovered, with an incredible wealth of sizes, forms and orbits.
Chemistry
The element lithium is the lightest solid element known the first pure sample of the salt of which was obtained by Swedish chemists Johan August Arfwedson and Jöns Jacob Berzelius in 1817. Lithium is a highly reactive element and has to be stored in oil to prevent it catching fire on exposure to moist air or water.
Lithium’s high reactivity is also its strength. It catches fire in moist air because it readily releases its outer electron, and this property is utilised in making the lithium battery. It was Stanley Whittingham of Binghamton University, State University of New York, USA, who developed the first functional lithium battery in the early 1970s. The light weight made it possible to pack a lot of lithium into a small space, unlike the large and heavy lead-acid batteries that dominated at the time. Whittingham put metallic lithium in one end and a layered material called titanium disulphide at the other; the titanium had spaces that could capture the flowing electrons. But battery with metallic lithium was too explosive to be viable. As the lithium battery with metallic lithium was repeatedly charged, thin whiskers of lithium grew from the lithium electrode. When they reached the other electrode, the battery short-circuited which often lead to an explosion.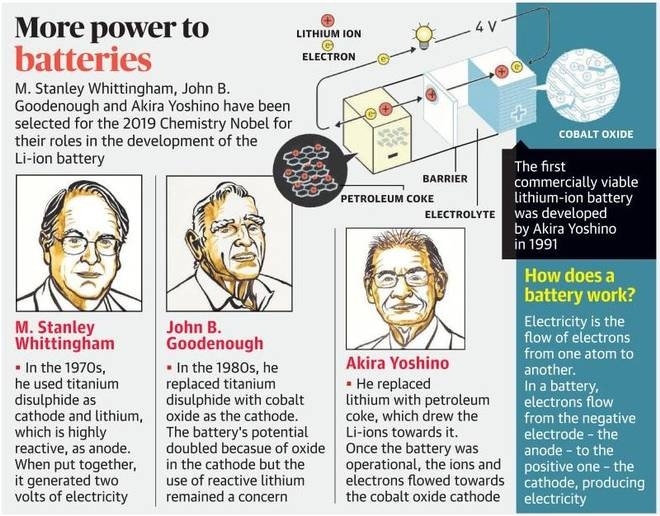
In 1980, John Goodenough of the University of Texas at Austin, USA, doubled the battery’s potential by using cobalt oxide in the lithium battery cathode, creating the right conditions for a vastly more powerful and useful battery. Then in 1985, Akira Yoshino of Asahi Kasei Corporation and Meijo University in Japan, succeeded in eliminating metallic lithium from the battery and developed a battery based entirely on lithium ions, which are safer than battery based on pure lithium metal. This made the battery much safer and workable in practice. Lithium-ion batteries are lightweight and powerful as compared with older battery technology and are ubiquitous today, used in cell phones, laptops, electric cars and renewable-energy storage devices that can help address the problems of climate change.
For their ground-breaking research on lithium-ion battery, Stanley Whittingham, John Goodenough and Akira Yoshino have been jointly awarded the 2019 Nobel Prize in Chemistry for their work on “the development of lithium ion batteries”. Aged 97, Goodenough is the oldest person to receive a Nobel Prize.
In modern lithium batteries, both the anode and cathode are made of layered materials that can store lithium ions in the gaps or spaces between their layers. When the battery is in use, electrons travel from the anode to the cathode through the external circuit – generating the current required to power whatever device the battery is connected to. At the same time, the positive lithium ions travel through the electrolyte from anode to cathode, where they are again stored. When the battery is recharged, the reverse process takes place with the electrons and lithium ions flowing back to the anode. 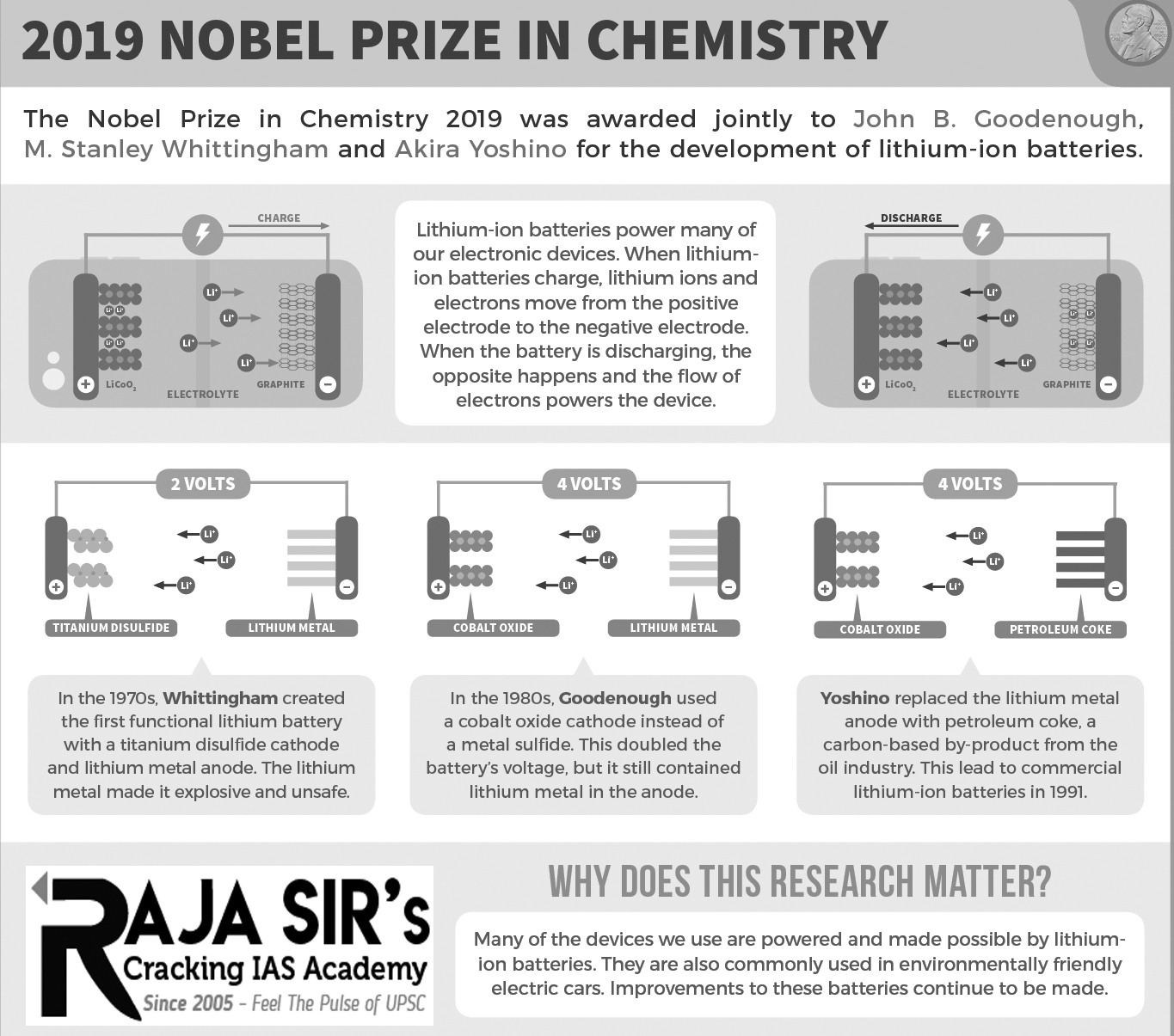
Physiology or Medicine
When we talk of life on Earth, we know how important oxygen is to our survival. Animals and humans need oxygen to convert food into useful energy. The fundamental question of how cells sense oxygen has implications for several biological processes 32 including development of embryo, cancer, stroke, diabetes, and other ischemic diseases. No wonder, this is an important scientific mystery that researchers have been trying to crack for many years.
Yet, despite the publication of hundreds of papers on this subject, till recently there was no clear consensus regarding what the cellular oxygen sensor is, or even the number of sensing mechanisms there might be. Now scientists seem to have solved the mystery.
The Nobel Prize in Physiology or Medicine for 2019 has been awarded to three scientists – cancer researcher William Kaelin of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, Massachusetts, USA; physician-scientist Peter Ratcliffe of the University of Oxford and the Francis Crick Institute, London, England; and geneticist Gregg Semenza of the Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, “for their discoveries of how cells sense and adapt to oxygen availability”. 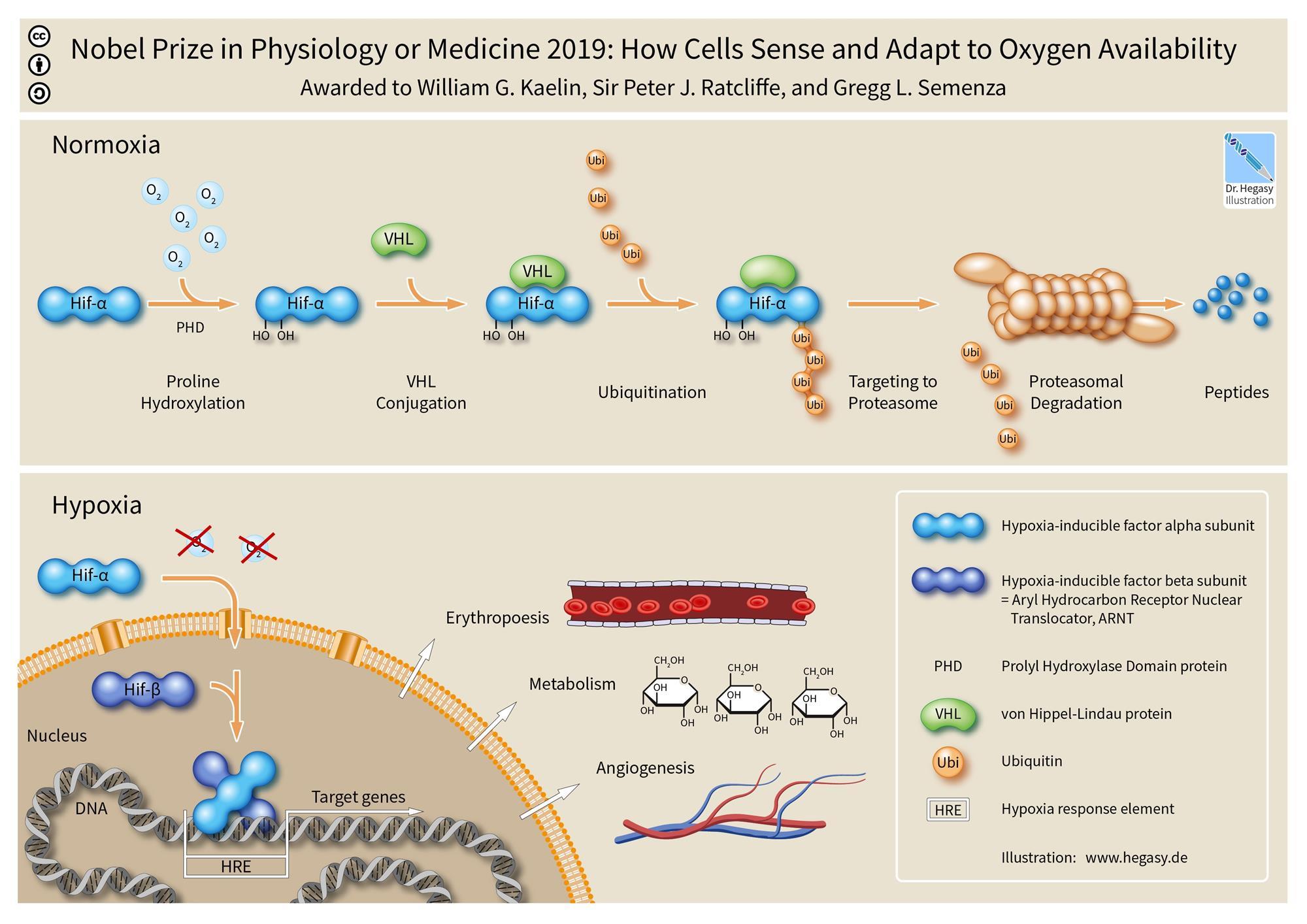
The body’s tissues can be deprived of oxygen during exercise or when blood flow is interrupted, such as during a stroke. Cells’ ability to sense oxygen is also crucial for the proper growth of a developing foetus and placenta, and also in tumour growth, because the mass of rapidly growing cells can deplete oxygen in the interior of a tumour.
During researches carried out in the 1990s, the three scientists, working independently, revealed the chain of molecular events that allow cells to detect and respond to different levels of oxygen. They had discovered the molecular processes that cells go through to respond to oxygen levels in the body. They found that central to this is a mechanism involving a protein complex called hypoxia-inducible factor (HIF) and a gene called VHL.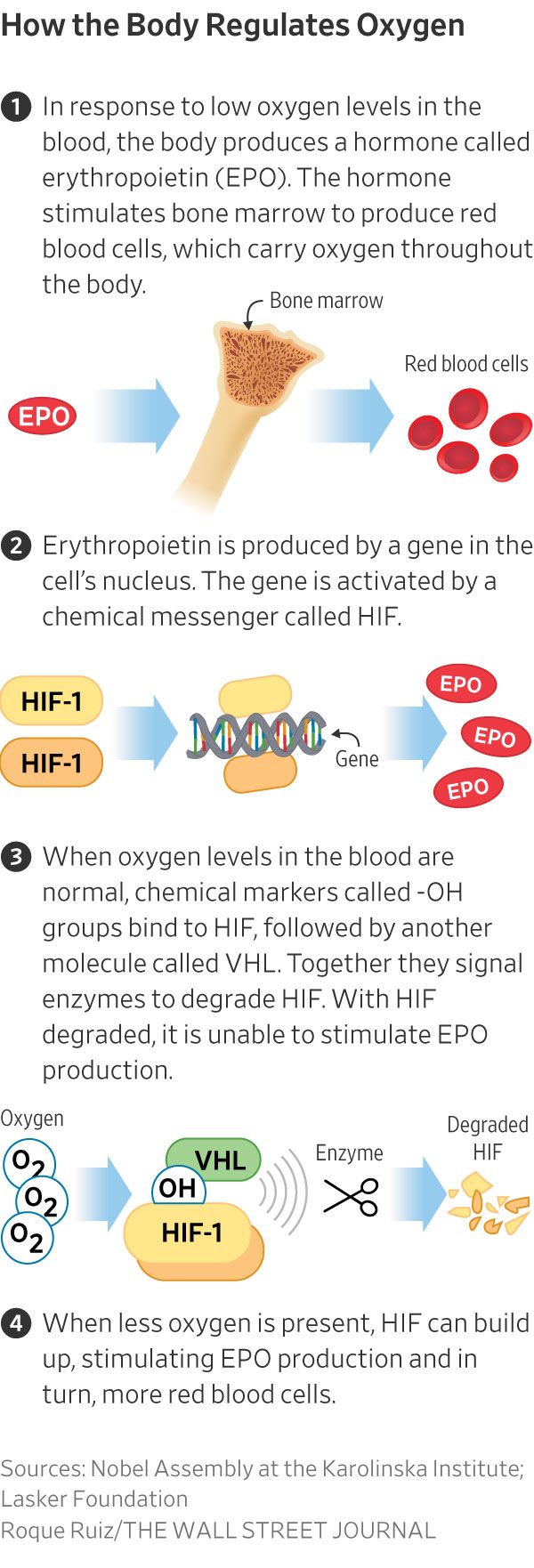
The work of the three scientists has helped researchers to understand how the body detects and adapts to low oxygen levels by, for example, making more red blood cells and growing new blood vessels. Their work has established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function. Their discoveries have also paved the way for promising new strategies to fight anaemia, cancer and many other diseases.
The work of Semenza and Ratcliffe concerned study of the regulation of a hormone called erythropoietin, which is crucial for stimulating the production of red blood cells in response to low levels of oxygen. Semenza and his team identified a pair of genes that encode the two proteins that form HIF and work together to turn on certain genes and boost erythropoietin production when oxygen is low.
Meanwhile, Kaelin’s work showed that the VHL gene may also be involved in how cells respond to oxygen, after studying a genetic syndrome called von HippelLindau’s disease. This genetic disease leads to dramatically increased risk of certain cancers in families with inherited VHL mutations.
Thanks to the ground-breaking work of the three Nobel Laureates, we know much more about how different oxygen levels regulate fundamental physiological processes. Oxygen sensing allows cells to adapt their metabolism to low oxygen levels: for example, in our muscles during intense exercise. Other examples of adaptive processes controlled by oxygen sensing include the generation of new blood vessels and the production of additional red blood cells. Our immune system and many other physiological functions are also fine-tuned by the oxygen-sensing machinery. Oxygen sensing has also been shown to be essential during foetal development for controlling normal blood vessel formation and placenta development.
The work has led researchers to develop drugs that target oxygen-sensing processes, including drugs for cancer. Drugs that prevent VHL from binding to HIF and causing its degradation are also being investigated as treatments for anaemia and renal failure. Chinese regulators approved the first of these drugs in 2018.









 Latest News
Latest News General Studies
General Studies