- Home
- Prelims
- Mains
- Current Affairs
- Study Materials
- Test Series
 EDITORIALS & ARTICLES
EDITORIALS & ARTICLES
Nitrogen Pollution
Nitrogen is the most abundant element in our atmosphere- approximately 78% of the atmosphere, hence is crucial to life. Nitrogen is found in soils and plants, in the water, and the air. It is also essential to life as a key building block of DNA, which determines our genetics, is essential to plant growth, and therefore necessary for the food we grow.
The balance of nitrogen is crucial for the environment and the life it sustains. When plants lack nitrogen, they become yellowed, with stunted growth, and produce smaller fruits and flowers. But when too much nitrogen is supplied it pollutes water systems and endangers aquatic life.
Nitrogen is a chief constituent of the bodies of living organisms as the Nitrogen atoms are found in all proteins, hormones, chlorophylls, vitamins, and DNA.
Plants and microbes compete for the limited nitrogen available in the soil- hence it acts as a limiting agent for both natural and agricultural ecosystems and exists in the atmosphere as N2.
Other sources of atmospheric nitrogen oxides are industrial combustions, forest fires, automobile exhaust, and power generating stations.
Nitrogen cycle:
The nitrogen cycle is a biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates through the atmosphere, and the terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes.
Stages of the nitrogen cycle:
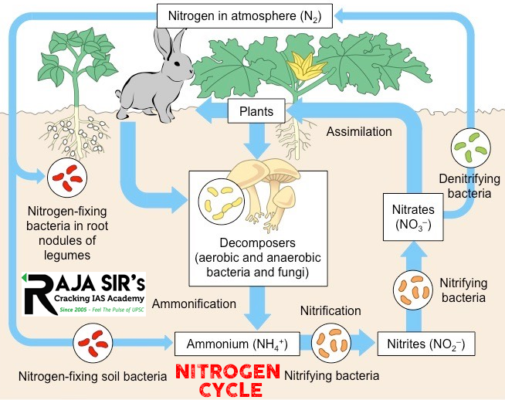 Effect of human activities of the nitrogen cycle
The excessive use of fertilizers for farming is one of the major causes of eutrophication in water systems. The agricultural runoff contains an uncontrolled amount of nutrients that cannot be balanced by the natural cycle.
Nitrogen gases and aerosols released by factories can be directly toxic to certain plant species, affecting the physiology and growth of plants near large sources of nitrogen pollution. Changes to plant species may also occur, as the accumulation of nitrogen compounds increases its availability in the ecosystem, eventually changing the species composition, plant diversity, and nitrogen cycle.
Reactive nitrogen from human activities can cause nitrate accumulation in the natural water environment, which can create harmful impacts on human health. Excessive use of N-fertilizer in agriculture has been one of the major sources of nitrate pollution in groundwater and surface water which can lead to nitrate poisoning.
Nitrogen pollution
Water Pollution
Effect of human activities of the nitrogen cycle
The excessive use of fertilizers for farming is one of the major causes of eutrophication in water systems. The agricultural runoff contains an uncontrolled amount of nutrients that cannot be balanced by the natural cycle.
Nitrogen gases and aerosols released by factories can be directly toxic to certain plant species, affecting the physiology and growth of plants near large sources of nitrogen pollution. Changes to plant species may also occur, as the accumulation of nitrogen compounds increases its availability in the ecosystem, eventually changing the species composition, plant diversity, and nitrogen cycle.
Reactive nitrogen from human activities can cause nitrate accumulation in the natural water environment, which can create harmful impacts on human health. Excessive use of N-fertilizer in agriculture has been one of the major sources of nitrate pollution in groundwater and surface water which can lead to nitrate poisoning.
Nitrogen pollution
Water Pollution
Soil Pollution
 South Asian Nitrogen Hub (SANH)
South Asian Nitrogen Hub (SANH)
- Nitrogen-fixing
- Nitrification
- Amonification
- Denitrification
- Nitrogen fixation:
- Nitrogen is usable only after it is fixed. Nitrogen fixation is a process where bacteria convert N2into ammonia, a form of nitrogen usable by plants.
- Non-symbiotic bacteria or free-living bacteria- Azobacter and Beijemickia (aerobic); Clostridium and Rhodosprillium (anaerobic).
- Symbiotic bacteria- Rhizobium– live in association with leguminous root nodule plants.
- Some cyanobacteria, blue-green algae (Nostoc, Anabaena, Spirulina) are major sources of nitrogen fixation in oceans.
- The lightning and UV radiation also provide enough energy to convert nitrogen to nitrogen oxides.
- The industrial process like fertilizer factories also accomplish nitrogen fixation,
- Nitrification: ammonia to nitrates
- Ammonium ions are directly taken up by some plants while most absorb nitrates obtained by oxidizing ammonia and ammonium ions.
- Ammonium ions are first oxidized to nitrite by Nitrosomonas/ Nitrococcus bacteria.
- Nitrite is then oxidized to nitrate by Nitrobacter bacteria (chemoautotrophs).
- Plants absorb these nitrates and convert them into amino acids.
- Ammonification: urea, uric acid to ammonia
- Living organisms produce nitrogenous waste products like urea and uric acid.
- These waste products and dead remains of the organisms are converted back into inorganic ammonia and ammonium ions by bacteria by ammonification.
- Denitrification: nitrate to nitrogen
- The process of reducing nitrate in the soil to nitrogen is called denitrification.
- Soil and oceans have denitrifying bacteria like Pseudomonas and Thiobacillus which convert nitrate/nitrites to elemental nitrogen.
- This nitrogen is released into the atmosphere completing the cycle.
 Effect of human activities of the nitrogen cycle
The excessive use of fertilizers for farming is one of the major causes of eutrophication in water systems. The agricultural runoff contains an uncontrolled amount of nutrients that cannot be balanced by the natural cycle.
Nitrogen gases and aerosols released by factories can be directly toxic to certain plant species, affecting the physiology and growth of plants near large sources of nitrogen pollution. Changes to plant species may also occur, as the accumulation of nitrogen compounds increases its availability in the ecosystem, eventually changing the species composition, plant diversity, and nitrogen cycle.
Reactive nitrogen from human activities can cause nitrate accumulation in the natural water environment, which can create harmful impacts on human health. Excessive use of N-fertilizer in agriculture has been one of the major sources of nitrate pollution in groundwater and surface water which can lead to nitrate poisoning.
Nitrogen pollution
Water Pollution
Effect of human activities of the nitrogen cycle
The excessive use of fertilizers for farming is one of the major causes of eutrophication in water systems. The agricultural runoff contains an uncontrolled amount of nutrients that cannot be balanced by the natural cycle.
Nitrogen gases and aerosols released by factories can be directly toxic to certain plant species, affecting the physiology and growth of plants near large sources of nitrogen pollution. Changes to plant species may also occur, as the accumulation of nitrogen compounds increases its availability in the ecosystem, eventually changing the species composition, plant diversity, and nitrogen cycle.
Reactive nitrogen from human activities can cause nitrate accumulation in the natural water environment, which can create harmful impacts on human health. Excessive use of N-fertilizer in agriculture has been one of the major sources of nitrate pollution in groundwater and surface water which can lead to nitrate poisoning.
Nitrogen pollution
Water Pollution
- Nitrogen compounds running off agricultural lands dissolve in rivers, lakes or groundwater and pollutes them.
- Creates harmful algal blooms and dead zones in oceans and algae produce toxins that are harmful to human and aquatic organisms.
- It indirectly affects fisheries and biodiversity in coastal areas as well.gree
- Drinking water is contaminated by excessive nitrate concentrations which has an adverse impact on human health such as reduce blood flow, cancer, and endemic goiters.
|
Eutrophication 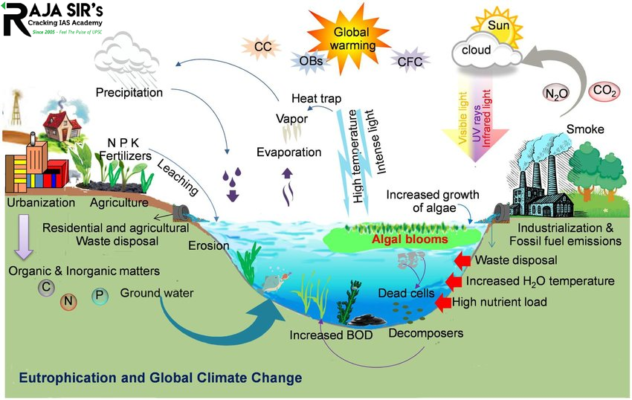 Excess nitrogen can also leach from the soil into underground water sources, entering aquatic systems as above-ground runoff. This excess nitrogen can build up, leading to a process called eutrophication.
When excess nitrogen enriches the water, it leads to unrestrained growth of plants and algae which block the light from reaching the bottom layers of the water system. This can even cause a lake to turn bright green or other colors, with an “algal bloom” of smelly algae called phytoplankton.
When these phytoplankton dies, microbes in the water decompose them and the process reduces the amount of dissolved oxygen in the water. This can lead to the formation of a “dead zone” that does not have enough oxygen to support most life forms eventually killing the organisms. These dead zones can form in freshwater lakes and also in coastal environments where rivers full of nutrients from agricultural runoff flow into oceans.
Excess nitrogen can also leach from the soil into underground water sources, entering aquatic systems as above-ground runoff. This excess nitrogen can build up, leading to a process called eutrophication.
When excess nitrogen enriches the water, it leads to unrestrained growth of plants and algae which block the light from reaching the bottom layers of the water system. This can even cause a lake to turn bright green or other colors, with an “algal bloom” of smelly algae called phytoplankton.
When these phytoplankton dies, microbes in the water decompose them and the process reduces the amount of dissolved oxygen in the water. This can lead to the formation of a “dead zone” that does not have enough oxygen to support most life forms eventually killing the organisms. These dead zones can form in freshwater lakes and also in coastal environments where rivers full of nutrients from agricultural runoff flow into oceans. |
- As the world needs to feed an ever-growing population, loss of arable land is a major global problem.
- Excessive use of nitrogen fertilizers causes soil acidification & soil nutrient depletion.
- The lowering pH as a result of the acidification causes nutrient disorders and increased toxicity in plants.
- It may also affect natural soil decomposition.
- Nitrogen emissions from industry, agriculture and vehicles cause air pollution.
- The release of nitrous oxide is essentially a greenhouse gas which is harmful to the environment.
- In 2017, a large team of Indian scientists had come out with The Indian Nitrogen Assessment (INA).
- India had become the third country/entity after the United States and the European Union to have assessed the environmental impact of nitrogen on their respective regions comprehensively.
- The INA shows that agriculture is the main source of nitrogen pollution in India. Within agriculture, cereals pollute the most.
- Rice and wheat take up the maximum cropped area in India at 36.95 million hectares (ha) and 26.69 million ha respectively.
- India consumes 17 Mt (million tonnes) of nitrogen fertiliser annually as per the data of the Fertiliser Association of India.
- Only 33 per cent of the nitrogen that is applied to rice and wheat through fertilisers is taken up by the plants in the form of nitrates (NO3). This is called Nitrogen Use Efficiency or NUE.
- The remaining 67 per cent remains in the soil and seeps into the surrounding environment, causing a cascade of environmental and health impacts.
- The Indian government is leading a resolution on nitrogen pollution in the UNEA in Nairobi that starts from this March 11.
- This is a historic event as India has never pushed for a resolution of such importance at any UN congregation before.
- And this has happened because India can now leverage its own nitrogen assessment and its strong support to South Asian and other regional assessments with a more inclusive approach.
- This would lead a process for faster global consensus and a more realistic programme of action.
- Nitrogen is an inert gas that’s necessary for life. But we’re changing it into forms that are harmful, overloading the environment with it, and throwing the natural nitrogen cycle out of whack.
- Nitrogen compounds running off farmland have led to water pollution problems around the world, while nitrogen emissions from industry, agriculture and vehicles make a big contribution to air pollution.
- Over 80% of the nitrogen in soil is not utilised by humans. While over four-fifths of the nitrogen is used to feed livestock, only about six per cent reaches humans in case of non-vegetarian diet, as compared to the 20% that reaches the plate of a vegetarian.
- Nitrogen becomes a pollutant when it escapes into the environment and reacts with other organic compounds. It is either released into the atmosphere, gets dissolved in water sources such as rivers, lakes or groundwater, or remains in the soil. While it might lead to favourable growth of species that can utilise this nutrient, nitrogen as a pollutant is often detrimental to the environment and health.
- It creates of harmful algal blooms and dead zones in our waterways and oceans; the algae produce toxins which are harmful to human and aquatic organisms (and indirectly affects fisheries and biodiversity in coastal areas).
- Contamination of drinking water. 10 million people in Europe are potentially exposed to drinking water with nitrate concentrations above recommended levels. This can have an adverse effect on human health.
- Food Security: Excessive nitrogen fertiliser application contributes to soil nutrient depletion. As the world needs to feed an ever growing population loss of arable land is major global problem.
- The release of Nitrous Oxide is essentially a greenhouse gas which is harmful to the environment.
 South Asian Nitrogen Hub (SANH)
South Asian Nitrogen Hub (SANH)
- The South Asian Nitrogen Hub (SANH) is a major international research programme to tackle the challenge that nitrogen pollution poses in South Asia.
- The SANH will be established with funding from UK Research and Innovation (UKRI) under its Global Challenges Research Fund (GCRF).
- 18 Indian research institutions are part of a group of 50 which have received £20 million funding from the United Kingdom Government.
- The SANH will study the impact of the different forms of pollution to form a coherent picture of the nitrogen cycle.
- In particular, it will look at nitrogen in agriculture in eight countries – India, Pakistan, Bangladesh, Nepal, Afghanistan, Sri Lanka, Bhutan and Maldives.
- Its recommendations will support cleaner and more profitable farming, as well as industrial recycling of nitrogen, fostering development of a cleaner circular economy for nitrogen.
International Nitrogen Initiative
INI is an international program, set up in 2003 under the sponsorship of the Scientific Committee on Problems of the Environment (SCOPE) and from the International Geosphere-Biosphere Program (IGBP). The key aims of the INI are to:
- optimize nitrogen’s beneficial role in sustainable food production, and
- minimize nitrogen’s negative effects on human health and the environment resulting from food and energy production.
- Nitrogen is crucial to food production in India, however, its excessive usage in agriculture has put us under serious risk.
- We cannot produce sufficient food to feed the entire nation without nitrogen, but at the same time, we cannot keep releasing a higher amount of nitrogen due to its polluting effects.
- Thus the challenge is to optimally utilize the nitrogen while reducing its negative impacts.
- Since the issue of nitrogen pollution starts to gain global attention, there have been innovations seeks to improve its efficiency by optimizing usage.
- A simpler method of minimizing nitrogen application in soil is Precision farming where small quantities of nitrogen are administered routinely rather than applying large doses uniformly across the field.
- Zero Budget Natural Farming which involves usage of locally available materials such as cow dung and cow urine to increase soil productivity and plant growth.
- Also, tablets and coated forms of nitrogen, when applies at the root level = release nutrients slowly to the crops.
- Notably, Bangladesh has managed to improve the efficiency of nutrient uptake by plants by applying fertilizers through tablets.
- A similar initiative has been taken in India with neem-coated urea.
- These methods combined with the organic fertilizers and optimal timing of application, sowing, and watering, have shown marked improvement over traditional efficiencies of nitrogen.









 Latest News
Latest News General Studies
General Studies